Predominant outer membrane antigens of Bartonella henselae
- PMID: 15155610
- PMCID: PMC415646
- DOI: 10.1128/IAI.72.6.3097-3105.2004
Predominant outer membrane antigens of Bartonella henselae
Abstract
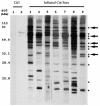
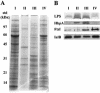
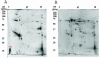
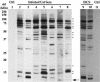
A hallmark of Bartonella henselae is persistent bacteremia in cats despite the presence of a vigorous host immune response. To understand better the long-term survival of B. henselae in cats, we examined the feline humoral immune response to B. henselae outer membrane (OM) proteins in naturally and experimentally infected cats. Initially, a panel of sera (n = 42) collected throughout North America from naturally infected cats was used to probe B. henselae total membranes to detect commonly recognized antigens. Twelve antigens reacted with sera from at least 85% of cats, and five were recognized by sera from all cats. To localize these antigens further, OMs were purified on discontinuous sucrose density step gradients. Each membrane fraction (OM, hybrid or inner membrane [IM]) contained less than 1% of the total malate dehydrogenase activity (soluble marker), indicating very little contamination by cytoplasmic proteins. FtsI, an integral IM cell division protein, was used to identify the low-density fraction (rho = 1.13 g/cm3) as putative IM (<5% of the total FtsI localized to the high-density fraction) while lipopolysaccharide (LPS) and Pap31, a homolog of the Bartonella quintana heme-binding protein A (HbpA), defined the high-density fraction (rho = 1.20 g/cm3) as putative OM. Additionally, little evidence of cross-contamination between the IM and OM was evident by two-dimensional gel electrophoresis. When purified OMs were probed with feline sera, antigenic proteins profiles were very similar to those observed with total membranes, indicating that many, but not all, of the immunoreactive proteins detected in the initial immunoblots were OM components. Interestingly, two-dimensional immunoblots indicated that B. henselae LPS and members of the Hbp family of proteins did not appear to stimulate an humoral response in any infected cats. Seven proteins were recognized by at least 70% of sera tested, but only three were recognized by all sera. Nanospray-tandem mass spectrometry was used to identify OM components, including the immunodominant OM proteins. Recognition of the nonimmunogenic nature of the major OM components, such as LPS, and identification of the predominant immunogens should elucidate the mechanisms by which B. henselae establishes persistent bacteremic infections within cats. Additionally, the common antigens may serve as potential feline vaccine candidates to eliminate the pathogen from its animal reservoir.
Figures
Similar articles
-
Experimental infection of specific pathogen free (SPF) cats with two different strains of bartonella henselae type I: a comparative study.Vet Res. 2002 Nov-Dec;33(6):669-84. doi: 10.1051/vetres:2002048. Vet Res. 2002. PMID: 12498568
-
Experimental infection of domestic cats with passaged genotype I Bartonella henselae.Vet Microbiol. 2007 Jun 21;122(3-4):290-7. doi: 10.1016/j.vetmic.2007.01.018. Epub 2007 Jan 31. Vet Microbiol. 2007. PMID: 17321078
-
Immunogenicity of Bartonella henselae P26 in cats.Vet Immunol Immunopathol. 2009 Dec 15;132(2-4):251-6. doi: 10.1016/j.vetimm.2009.05.005. Epub 2009 May 18. Vet Immunol Immunopathol. 2009. PMID: 19500857
-
[Bartonella henselae and its infections].Mikrobiyol Bul. 2008 Jan;42(1):163-75. Mikrobiyol Bul. 2008. PMID: 18444576 Review. Turkish.
-
[Prevalence of Bartonella henselae in stray and domestic cats in different Italian areas: evaluation of the potential risk of transmission of Bartonella to humans].Parassitologia. 2004 Jun;46(1-2):127-9. Parassitologia. 2004. PMID: 15305701 Review. Italian.
Cited by
-
Hemin-binding proteins as potent markers for serological diagnosis of infections with Bartonella quintana.Clin Vaccine Immunol. 2013 Apr;20(4):620-6. doi: 10.1128/CVI.00717-12. Epub 2013 Feb 13. Clin Vaccine Immunol. 2013. PMID: 23408526 Free PMC article.
-
Heme binding proteins of Bartonella henselae are required when undergoing oxidative stress during cell and flea invasion.PLoS One. 2012;7(10):e48408. doi: 10.1371/journal.pone.0048408. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144761 Free PMC article.
-
Immunogenicity of trimeric autotransporter adhesins and their potential as vaccine targets.Med Microbiol Immunol. 2020 Jun;209(3):243-263. doi: 10.1007/s00430-019-00649-y. Epub 2019 Dec 1. Med Microbiol Immunol. 2020. PMID: 31788746 Free PMC article. Review.
-
American Association of Feline Practitioners 2006 Panel report on diagnosis, treatment, and prevention of Bartonella spp. infections.J Feline Med Surg. 2006 Aug;8(4):213-26. doi: 10.1016/j.jfms.2006.05.006. J Feline Med Surg. 2006. PMID: 16846781 Free PMC article. No abstract available.
-
P26-based serodiagnosis for Bartonella spp. infection in cats.Comp Med. 2008 Aug;58(4):375-80. Comp Med. 2008. PMID: 18724780 Free PMC article.
References
-
- Abbott, R. C., B. B. Chomel, R. W. Kasten, K. A. Floyd-Hawkins, Y. Kikuchi, J. E. Koehler, and N. C. Pedersen. 1997. Experimental and natural infection with Bartonella henselae in domestic cats. Comp. Immunol. Microbiol. Infect. Dis. 20:41-51. - PubMed
-
- Burgess, A. W., and B. E. Anderson. 1998. Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb. Pathog. 25:157-164. - PubMed
-
- Chenoweth, M. R., G. A. Somerville, D. C. Krause, K. L. O'Reilly, and F. C. Gherardini. 2004. Growth characteristics of Bartonella henselae in a novel liquid medium: primary isolation, growth-phase-dependent phage induction, and metabolic studies. Appl. Environ. Microbiol. 70:656-663. - PMC - PubMed
-
- Chomel, B. B., R. C. Abbott, R. W. Kasten, K. A. Floyd-Hawkins, P. H. Kass, C. A. Glaser, N. C. Pedersen, and J. E. Koehler. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33:2445-2450. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

