Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract
- PMID: 15155618
- PMCID: PMC415693
- DOI: 10.1128/IAI.72.6.3171-3178.2004
Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract
Abstract
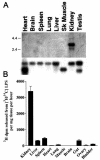
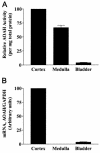
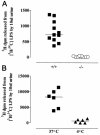
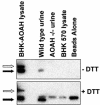
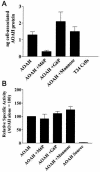
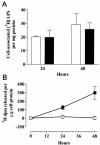
Acyloxyacyl hydrolase (AOAH) is an unusual but highly conserved lipase, previously described only in myeloid cells, that removes secondary fatty acyl chains from bacterial lipopolysaccharides (LPS) and may also act on various glycero(phospho)lipids. Deacylation by AOAH greatly reduces the ability of LPS to stimulate cells via CD14-MD-2-Toll-like receptor 4. We report here that renal cortical tubule cells produce AOAH and secrete it into urine, where it can deacylate LPS. In vitro studies revealed that proximal tubule cells secrete pro-AOAH, which can be taken up by bladder cells and processed to the heterodimeric, more enzymatically active, mature form of AOAH. AOAH can then be used by the recipient cells to deacylate LPS. The enzyme produced by proximal tubule epithelium may thus be shared with downstream cells. In addition, mature AOAH is found in the urine. We suggest that cortical tubule cells may produce and secrete AOAH to limit inflammatory responses to gram-negative bacteria throughout the urinary tract.
Figures

References
-
- Bäckhed, F., M. Söderhäll, P. Ekmann, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell. Microbiol. 3:153-158. - PubMed
-
- Donnenberg, M. S., B. Newman, S. J. Utsalo, A. L. Trifillis, J. R. Hebel, and J. W. Warren. 1994. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J. Infect. Dis. 169:831-838. - PubMed
-
- Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

