Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages
- PMID: 15155622
- PMCID: PMC415696
- DOI: 10.1128/IAI.72.6.3204-3217.2004
Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages
Abstract
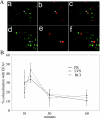
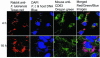
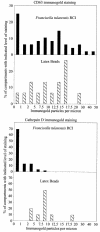
Francisella tularensis, the agent of tularemia, is an intracellular pathogen, but little is known about the compartment in which it resides in human macrophages. We have examined the interaction of a recent virulent clinical isolate of F. tularensis subsp. tularensis and the live vaccine strain with human macrophages by immunoelectron and confocal immunofluorescence microscopy. We assessed the maturation of the F. tularensis phagosome by examining its acquisition of the lysosome-associated membrane glycoproteins (LAMPs) CD63 and LAMP1 and the acid hydrolase cathepsin D. Two to four hours after infection, vacuoles containing live F. tularensis cells acquired abundant staining for LAMPs but little or no staining for cathepsin D. However, after 4 h, the colocalization of LAMPs with live F. tularensis organisms declined dramatically. In contrast, vacuoles containing formalin-killed bacteria exhibited intense staining for all of these late endosomal/lysosomal markers at all time points examined (1 to 16 h). We examined the pH of the vacuoles 3 to 4 h after infection by quantitative immunogold staining and by fluorescence staining for lysosomotropic agents. Whereas phagosomes containing killed bacteria stained intensely for these agents, indicating a marked acidification of the phagosomes (pH 5.5), phagosomes containing live F. tularensis did not concentrate these markers and thus were not appreciably acidified (pH 6.7). An ultrastructural analysis of the F. tularensis compartment revealed that during the first 4 h after uptake, the majority of F. tularensis bacteria reside within phagosomes with identifiable membranes. The cytoplasmic side of the membranes of approximately 50% of these phagosomes was coated with densely staining fibrils of approximately 30 nm in length. In many cases, these coated phagosomal membranes appeared to bud, vesiculate, and fragment. By 8 h after infection, the majority of live F. tularensis bacteria lacked any ultrastructurally discernible membrane separating them from the host cell cytoplasm. These results indicate that F. tularensis initially enters a nonacidified phagosome with LAMPs but without cathepsin D and that the phagosomal membrane subsequently becomes morphologically disrupted, allowing the bacteria to gain direct access to the macrophagic cytoplasm. The capacity of F. tularensis to alter the maturation of its phagosome and to enter the cytoplasm is likely an important element of its capacity to parasitize macrophages and has major implications for vaccine development.
Figures

References
-
- Bell, J. F., C. R. Owen, and C. L. Larson. 1955. Virulence of Bacterium tularense. I. A study of the virulence of Bacterium tularense in mice, guinea pigs, and rabbits. J. Infect. Dis. 97:162-166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

