Recombinant Streptococcus equi proteins protect mice in challenge experiments and induce immune response in horses
- PMID: 15155624
- PMCID: PMC415648
- DOI: 10.1128/IAI.72.6.3228-3236.2004
Recombinant Streptococcus equi proteins protect mice in challenge experiments and induce immune response in horses
Abstract
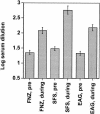
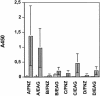
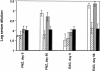
Horses that have undergone infection caused by Streptococcus equi subspecies equi (strangles) were found to have significantly increased serum antibody titers against three previously characterized proteins, FNZ (cell surface-bound fibronectin binding protein), SFS (secreted fibronectin binding protein), and EAG (alpha2-macroglobulin, albumin, and immunoglobulin G [IgG] binding protein) from S. equi. To assess the protective efficacy of vaccination with these three proteins, a mouse model of equine strangles was utilized. Parts of the three recombinant proteins were used to immunize mice, either subcutaneously or intranasally, prior to nasal challenge with S. equi subsp. equi. The adjuvant used was EtxB, a recombinant form of the B subunit of Escherichia coli heat-labile enterotoxin. It was shown that nasal colonization of S. equi subsp. equi and weight loss due to infection were significantly reduced after vaccination compared with a mock-vaccinated control group. This effect was more pronounced after intranasal vaccination than after subcutaneous vaccination; nearly complete eradication of nasal colonization was obtained after intranasal vaccination (P < 0.001). When the same antigens were administered both intranasally and subcutaneously to healthy horses, significant mucosal IgA and serum IgG antibody responses against FNZ and EAG were obtained. The antibody response was enhanced when EtxB was used as an adjuvant. No adverse effects of the antigens or EtxB were observed. Thus, FNZ and EAG in conjunction with EtxB are promising candidates for an efficacious and safe vaccine against strangles.
Figures






References
-
- Chanter, N. 2002. Bacterial infections including mycoplasmas. In P. Lekeux (ed.), Equine respiratory diseases. International Veterinary Information Services, Ithaca, N.Y.
-
- Chanter, N., K. C. Smith, and J. A. Mumford. 1995. Equine strangles modelled in mice. Vet. Microbiol. 43:209-218. - PubMed
-
- Hamlen, H. J., J. F. Timoney, and R. J. Bell. 1994. Epidemiologic and immunologic characteristics of Streptococcus equi infection in foals. JAVMA 204:768-775. - PubMed
-
- Harrington, D. J., I. C. Sutcliffe, and N. Chanter. 2002. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 4:501-510. - PubMed
-
- Jacobs, A. A., D. Goovaerts, P. J. Nuijten, R. P. Theelen, O. M. Hartford, and T. J. Foster. 2000. Investigations towards an efficacious and safe strangles vaccine: submucosal vaccination with a live attenuated Streptococcus equi. Vet. Rec. 147:563-567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

