Bacterial probiotic modulation of dendritic cells
- PMID: 15155633
- PMCID: PMC415669
- DOI: 10.1128/IAI.72.6.3299-3309.2004
Bacterial probiotic modulation of dendritic cells
Abstract
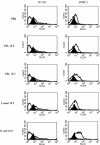
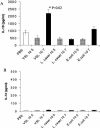
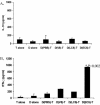
Intestinal dendritic cells are continually exposed to ingested microorganisms and high concentrations of endogenous bacterial flora. These cells can be activated by infectious agents and other stimuli to induce T-cell responses and to produce chemokines which recruit other cells to the local environment. Bacterial probiotics are of increasing use against intestinal disorders such as inflammatory bowel disease. They act as nonpathogenic stimuli within the gut to regain immunologic quiescence. This study was designed to determine the ability of a bacterial probiotic cocktail VSL#3 to alter cell surface antigen expression and cytokine production in bone marrow-derived dendritic cell-enriched populations. Cell surface phenotype was monitored by monoclonal fluorescent antibody staining, and cytokine levels were quantitated by enzyme-linked immunosorbent assay. High-dose probiotic upregulated the expression of C80, CD86, CD40, and major histocompatibility complex class II I-Ad. Neither B7-DC or B7RP-1 was augmented after low-dose probiotic or Lactobacillus casei treatment, but B7RP-1 showed increased expression on dendritic cells stimulated with the gram-negative bacterium Escherichia coli. Functional studies showed that probiotic did not enhance the ability of dendritic cells to induce allogeneic T-cell proliferation, as was observed for E. coli. Substantial enhancement of interleukin-10 release was observed in dendritic cell-enriched culture supernatants after 3 days of probiotic stimulation. These results demonstrate that probiotics possess the ability to modulate dendritic cell surface phenotype and cytokine release in granulocyte-macrophage colony-stimulating factor-stimulated bone marrow-derived dendritic cells. Regulation of dendritic cell cytokines by probiotics may contribute to the benefit of these molecules in treatment of intestinal diseases.
Figures




References
-
- Annuk, H., J. Shchepetova, T. Kullisaar, E. Songisepp, M. Zilmer, and M. Mikelsaar. 2003. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 94:403-412. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

