Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase
- PMID: 15155643
- PMCID: PMC415710
- DOI: 10.1128/IAI.72.6.3373-3382.2004
Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase
Abstract
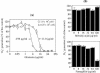
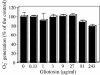
Reactive oxygen species are a critical weapon in the killing of Aspergillus fumigatus by polymorphonuclear leukocytes (PMN), as demonstrated by severe aspergillosis in chronic granulomatous disease. In the present study, A. fumigatus-produced mycotoxins (fumagillin, gliotoxin [GT], and helvolic acid) are examined for their effects on the NADPH oxidase activity in human PMN. Of these mycotoxins, only GT significantly and stoichiometrically inhibits phorbol myristate acetate (PMA)-stimulated O2- generation, while the other two toxins are ineffective. The inhibition is dependent on the disulfide bridge of GT, which interferes with oxidase activation but not catalysis of the activated oxidase. Specifically, GT inhibits PMA-stimulated events: p47phox phosphorylation, its incorporation into the cytoskeleton, and the membrane translocation of p67phox, p47phox, and p40phox, which are crucial steps in the assembly of the active NADPH oxidase. Thus, damage to p47phox phosphorylation is likely a key to inhibiting NADPH oxidase activation. GT does not inhibit the membrane translocation of Rac2. The inhibition of p47phox phosphorylation is due to the defective membrane translocation of protein kinase C (PKC) betaII rather than an effect of GT on PKC betaII activity, suggesting a failure of PKC betaII to associate with the substrate, p47phox, on the membrane. These results suggest that A. fumigatus may confront PMN by inhibiting the assembly of the NADPH oxidase with its hyphal product, GT.
Figures




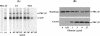
Similar articles
-
Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase.Infect Immun. 2005 Jan;73(1):235-44. doi: 10.1128/IAI.73.1.235-244.2005. Infect Immun. 2005. PMID: 15618159 Free PMC article.
-
Protein kinase C (PKC) isoforms translocate to Triton-insoluble fractions in stimulated human neutrophils: correlation of conventional PKC with activation of NADPH oxidase.J Immunol. 1999 Oct 15;163(8):4574-82. J Immunol. 1999. PMID: 10510401
-
Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid.J Leukoc Biol. 2006 Jan;79(1):223-34. doi: 10.1189/jlb.0705371. Epub 2005 Nov 7. J Leukoc Biol. 2006. PMID: 16275890
-
Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation.J Leukoc Biol. 2005 Nov;78(5):1025-42. doi: 10.1189/jlb.0804442. Epub 2005 Oct 4. J Leukoc Biol. 2005. PMID: 16204621 Review.
-
Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane.Semin Immunopathol. 2008 Jul;30(3):279-89. doi: 10.1007/s00281-008-0118-3. Epub 2008 Jun 7. Semin Immunopathol. 2008. PMID: 18536919 Review.
Cited by
-
The dehydrogenase region of the NADPH oxidase component Nox2 acts as a protein disulfide isomerase (PDI) resembling PDIA3 with a role in the binding of the activator protein p67 (phox.).Front Chem. 2015 Feb 4;3:3. doi: 10.3389/fchem.2015.00003. eCollection 2015. Front Chem. 2015. PMID: 25699251 Free PMC article.
-
Sensing the threat posed by Aspergillus infection.Curr Opin Microbiol. 2020 Dec;58:47-55. doi: 10.1016/j.mib.2020.08.004. Epub 2020 Sep 6. Curr Opin Microbiol. 2020. PMID: 32898768 Free PMC article. Review.
-
Epipolythiodioxopiperazine-Based Natural Products: Building Blocks, Biosynthesis and Biological Activities.Chembiochem. 2022 Dec 5;23(23):e202200341. doi: 10.1002/cbic.202200341. Epub 2022 Sep 15. Chembiochem. 2022. PMID: 35997236 Free PMC article. Review.
-
Aspergillus fumigatus Fumagillin Contributes to Host Cell Damage.J Fungi (Basel). 2021 Nov 3;7(11):936. doi: 10.3390/jof7110936. J Fungi (Basel). 2021. PMID: 34829223 Free PMC article.
-
An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes.Virulence. 2013 Oct 1;4(7):597-603. doi: 10.4161/viru.25906. Epub 2013 Aug 6. Virulence. 2013. PMID: 23921374 Free PMC article. Review.
References
-
- Akard, L. P., D. English, and T. G. Gabig. 1988. Rapid deactivation of NADPH oxidase in neutrophils: continuous replacement by newly activated enzyme sustains the respiratory burst. Blood 72:322-327. - PubMed
-
- Arruda, L. K., B. J. Mann, and M. D. Chapman. 1992. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J. Immunol. 149:3354-3359. - PubMed
-
- Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. - PubMed
-
- Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

