Mitogenicity of the recombinant mycobacterial 27-kilodalton lipoprotein is not connected to its antiprotective effect
- PMID: 15155644
- PMCID: PMC415711
- DOI: 10.1128/IAI.72.6.3383-3390.2004
Mitogenicity of the recombinant mycobacterial 27-kilodalton lipoprotein is not connected to its antiprotective effect
Abstract
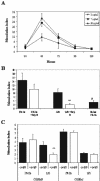
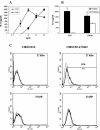
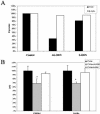
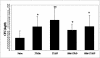
We reported previously that even though immunization with the recombinant mycobacterial 27-kDa lipoprotein (r27) induced a Th1-type response in mice, the vaccinated mice became more susceptible to challenge with Mycobacterium tuberculosis. In this study we show that r27 stimulates naive splenocytes to proliferate. Acylation of r27 was crucial for this effect, since a nonacylated mutant of r27, termed r27DeltaSP, failed to stimulate splenocytes either in vitro or in vivo. Depletion experiments indicated that only B cells were proliferating in a T-cell-independent manner. We also found that r27 is recognized by TLR2, which is involved in mitogenic stimulation. Interestingly, r27 but not r27DeltaSP induced high gamma interferon levels in splenocyte supernatants, whereas no significant interleukin-2 levels were detected. Since B-cell polyclonal activation might aggravate pathogen infection, we asked whether the antiprotective effect of the r27 lipoprotein is associated with its mitogenicity. We showed that, as in the case of r27, immunization of mice with the nonmitogenic r27DeltaSP lipoprotein resulted in increased M. tuberculosis multiplication. We conclude that the antiprotective effect of the r27 lipoprotein must be linked to properties of the polypeptide portion of the lipoprotein rather than to its lipid moiety and its mitogenicity.
Figures



Similar articles
-
Pseudo-rationale design of efficient TB vaccines: lesson from the mycobacterial 27-kDa lipoprotein.Tuberculosis (Edinb). 2006 May-Jul;86(3-4):225-35. doi: 10.1016/j.tube.2006.01.012. Epub 2006 Mar 3. Tuberculosis (Edinb). 2006. PMID: 16515885
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis.J Immunol. 2009 Aug 15;183(4):2659-68. doi: 10.4049/jimmunol.0900947. Epub 2009 Jul 20. J Immunol. 2009. PMID: 19620314
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
A toll for T cell costimulation.Ann Rheum Dis. 2004 Nov;63 Suppl 2(Suppl 2):ii76-ii78. doi: 10.1136/ard.2004.028308. Ann Rheum Dis. 2004. PMID: 15479878 Free PMC article. Review. No abstract available.
Cited by
-
Potentiating effects of MPL on DSPC bearing cationic liposomes promote recombinant GP63 vaccine efficacy: high immunogenicity and protection.PLoS Negl Trop Dis. 2011 Dec;5(12):e1429. doi: 10.1371/journal.pntd.0001429. Epub 2011 Dec 20. PLoS Negl Trop Dis. 2011. PMID: 22206029 Free PMC article.
-
Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis.J Clin Immunol. 2009 Jan;29(1):46-56. doi: 10.1007/s10875-008-9222-3. Epub 2008 Aug 9. J Clin Immunol. 2009. PMID: 18690522
-
Lipoproteins of bacterial pathogens.Infect Immun. 2011 Feb;79(2):548-61. doi: 10.1128/IAI.00682-10. Epub 2010 Oct 25. Infect Immun. 2011. PMID: 20974828 Free PMC article. Review.
-
Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs.Can J Vet Res. 2006 Jul;70(3):176-82. Can J Vet Res. 2006. PMID: 16850939 Free PMC article.
-
Gamma interferon and monophosphoryl lipid A-trehalose dicorynomycolate are efficient adjuvants for Mycobacterium tuberculosis multivalent acellular vaccine.Infect Immun. 2005 Jan;73(1):250-7. doi: 10.1128/IAI.73.1.250-257.2005. Infect Immun. 2005. PMID: 15618161 Free PMC article.
References
-
- Abed, N. S., J. H. Chace, A. L. Fleming, and J. S. Cowdery. 1994. Interferon-gamma regulation of B lymphocyte differentiation: activation of B cells is a prerequisite for IFN-γ-mediated inhibition of B cell differentiation. Cell. Immunol. 153:356-366. - PubMed
-
- Akkoyunlu, M., A. Melhus, C. Capiau, O. van Opstal, and A. Forsgren. 1997. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect. Immun. 65:5010-5016. - PMC - PubMed
-
- BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2:425-431. - PubMed
-
- Bigi, F., C. Espitia, A. Alito, M. Zumarraga, M. I. Romano, S. Cravero, and A. Cataldi. 1997. A novel 27 kDa lipoprotein antigen from Mycobacterium bovis. Microbiology 143:3599-3605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

