The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides
- PMID: 15155667
- PMCID: PMC415678
- DOI: 10.1128/IAI.72.6.3577-3583.2004
The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides
Abstract
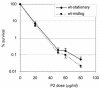
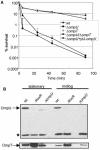
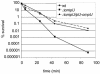
BPI (bactericidal/permeability-increasing) is a potent antimicrobial protein that was recently reported to be expressed as a surface protein on human gastrointestinal tract epithelial cells. In this study, we investigated the resistance of Vibrio cholerae, a small-bowel pathogen that causes cholera, to a BPI-derived peptide, P2. Unlike in Escherichia coli and Salmonella enterica serovar Typhimurium, resistance to P2 in V. cholerae was not dependent on the BipA GTPase. Instead, we found that ToxR, the master regulator of V. cholerae pathogenicity, controlled resistance to P2 by regulating the production of the outer membrane protein OmpU. Both toxR and ompU mutants were at least 100-fold more sensitive to P2 than were wild-type cells. OmpU also conferred resistance to polymyxin B sulfate, suggesting that this porin may impart resistance to cationic antibacterial proteins via a common mechanism. Studies of stationary-phase cells revealed that the ToxR-repressed porin OmpT may also contribute to P2 resistance. Finally, although the mechanism of porin-mediated resistance to antimicrobial peptides remains elusive, our data suggest that the BPI peptide sensitivity of OmpU-deficient V. cholerae is not attributable to a generally defective outer membrane.
Figures

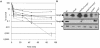
Similar articles
-
Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence.J Bacteriol. 2001 Jun;183(12):3652-62. doi: 10.1128/JB.183.12.3652-3662.2001. J Bacteriol. 2001. PMID: 11371530 Free PMC article.
-
Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization.Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):10220-4. doi: 10.1073/pnas.170219997. Proc Natl Acad Sci U S A. 2000. PMID: 10944196 Free PMC article.
-
Antimicrobial peptides activate the Vibrio cholerae sigmaE regulon through an OmpU-dependent signalling pathway.Mol Microbiol. 2007 Feb;63(3):848-58. doi: 10.1111/j.1365-2958.2006.05544.x. Epub 2006 Dec 20. Mol Microbiol. 2007. PMID: 17181782
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
-
Structure, regulation, and host interaction of outer membrane protein U (OmpU) of Vibrio species.Microb Pathog. 2022 Jan;162:105267. doi: 10.1016/j.micpath.2021.105267. Epub 2021 Oct 27. Microb Pathog. 2022. PMID: 34718127 Review.
Cited by
-
Outer membrane vesicles and the outer membrane protein OmpU govern Vibrio cholerae biofilm matrix assembly.mBio. 2024 Feb 14;15(2):e0330423. doi: 10.1128/mbio.03304-23. Epub 2024 Jan 11. mBio. 2024. PMID: 38206049 Free PMC article.
-
Ambient pH Alters the Protein Content of Outer Membrane Vesicles, Driving Host Development in a Beneficial Symbiosis.J Bacteriol. 2019 Sep 20;201(20):e00319-19. doi: 10.1128/JB.00319-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31331976 Free PMC article.
-
Mechanisms of the evolutionary arms race between Vibrio cholerae and Vibriophage clinical isolates.Int Microbiol. 2017 Sep;20(3):116-120. doi: 10.2436/20.1501.01.292. Int Microbiol. 2017. PMID: 29446802 Free PMC article. Review.
-
In vitro and in vivo antimicrobial activity of granulysin-derived peptides against Vibrio cholerae.J Antimicrob Chemother. 2008 May;61(5):1103-9. doi: 10.1093/jac/dkn058. Epub 2008 Feb 29. J Antimicrob Chemother. 2008. PMID: 18310138 Free PMC article.
-
Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8722-7. doi: 10.1073/pnas.1201313109. Epub 2012 May 15. Proc Natl Acad Sci U S A. 2012. PMID: 22589301 Free PMC article.
References
-
- Barker, H. C., N. Kinsella, A. Jaspe, T. Friedrich, and C. D. O'Connor. 2000. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol. Microbiol. 35:1518-1529. - PubMed
-
- Beamer, L. J., S. F. Carroll, and D. Eisenberg. 1997. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 276:1861-1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

