Legionella dumoffii DjlA, a member of the DnaJ family, is required for intracellular growth
- PMID: 15155669
- PMCID: PMC415686
- DOI: 10.1128/IAI.72.6.3592-3603.2004
Legionella dumoffii DjlA, a member of the DnaJ family, is required for intracellular growth
Abstract
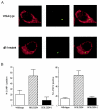
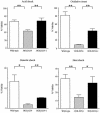
Legionella dumoffii is one of the common causes of Legionnaires' disease and is capable of replicating in macrophages. To understand the mechanism of survival within macrophages, transposon mutagenesis was employed to isolate the genes necessary for intracellular growth. We identified four defective mutants after screening 790 transposon insertion mutants. Two transposon insertions were in genes homologous to icmB or dotC, within dot/icm loci, required for intracellular multiplication of L. pneumophila. The third was in a gene whose product is homologous to the 17-kDa antigen forming part of the VirB/VirD4 type IV secretion system of Bartonella henselae. The fourth was in the djlA (for "dnaj-like A") gene. DjlA is a member of the DnaJ/Hsp40 family. Transcomplementation of the djlA mutant restored the parental phenotype in J774 macrophages, A549 human alveolar epithelial cells, and the amoeba Acanthamoeba culbertsoni. Using confocal laser-scanning microscopy and transmission electron microscopy, we revealed that in contrast to the wild-type strain, L. dumoffii djlA mutant-containing phagosomes were unable to inhibit phagosome-lysosome fusion. Transmission electron microscopy also showed that in contrast to the virulent parental strain, the djlA mutant was not able to recruit host cell rough endoplasmic reticulum. Furthermore, the stationary-phase L. dumoffii djlA mutants were more susceptible to H2O2, high osmolarity, high temperature, and low pH than was their parental strain. These results indicate that DjlA is required for intracellular growth and organelle trafficking, as well as bacterial resistance to environmental stress. This is the first report demonstrating that a single DjlA-deficient mutant exhibits a distinct phenotype.
Figures
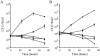
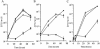



Similar articles
-
Characterization of the icmH and icmF genes required for Legionella pneumophila intracellular growth, genes that are present in many bacteria associated with eukaryotic cells.Infect Immun. 2004 Jun;72(6):3398-409. doi: 10.1128/IAI.72.6.3398-3409.2004. Infect Immun. 2004. PMID: 15155646 Free PMC article.
-
Characterization of Legionella pneumophila pmiA, a gene essential for infectivity of protozoa and macrophages.Infect Immun. 2005 Oct;73(10):6272-82. doi: 10.1128/IAI.73.10.6272-6282.2005. Infect Immun. 2005. PMID: 16177298 Free PMC article.
-
Differences in protein synthesis between wild type and intracellular growth-deficient strains of Legionella pneumophila in U937 and Acanthamoeba polyphaga.Microb Pathog. 2006 Apr;40(4):161-70. doi: 10.1016/j.micpath.2005.12.005. Epub 2006 Feb 20. Microb Pathog. 2006. PMID: 16488572
-
Intracellular parasitism and molecular determinants of Legionella virulence.Int Microbiol. 1999 Sep;2(3):145-54. Int Microbiol. 1999. PMID: 10943407 Review.
-
Subversion of a young phagosome: the survival strategies of intracellular pathogens.Cell Microbiol. 2000 Oct;2(5):365-77. doi: 10.1046/j.1462-5822.2000.00066.x. Cell Microbiol. 2000. PMID: 11207592 Review. No abstract available.
Cited by
-
Heat Shock Protein DnaJ in Pseudomonas aeruginosa Affects Biofilm Formation via Pyocyanin Production.Microorganisms. 2020 Mar 12;8(3):395. doi: 10.3390/microorganisms8030395. Microorganisms. 2020. PMID: 32178243 Free PMC article.
-
Legionella feeleii: pneumonia or Pontiac fever? Bacterial virulence traits and host immune response.Med Microbiol Immunol. 2019 Feb;208(1):25-32. doi: 10.1007/s00430-018-0571-0. Epub 2018 Nov 1. Med Microbiol Immunol. 2019. PMID: 30386929 Review.
-
Secretion of GOB metallo-beta-lactamase in Escherichia coli depends strictly on the cooperation between the cytoplasmic DnaK chaperone system and the Sec machinery: completion of folding and Zn(II) ion acquisition occur in the bacterial periplasm.Antimicrob Agents Chemother. 2009 Jul;53(7):2908-17. doi: 10.1128/AAC.01637-08. Epub 2009 May 11. Antimicrob Agents Chemother. 2009. PMID: 19433552 Free PMC article.
-
bdhA-patD operon as a virulence determinant, revealed by a novel large-scale approach for identification of Legionella pneumophila mutants defective for amoeba infection.Appl Environ Microbiol. 2009 Jul;75(13):4506-15. doi: 10.1128/AEM.00187-09. Epub 2009 May 1. Appl Environ Microbiol. 2009. PMID: 19411431 Free PMC article.
-
Screening and identification of DnaJ interaction proteins in Streptococcus pneumoniae.Curr Microbiol. 2013 Dec;67(6):732-41. doi: 10.1007/s00284-013-0424-4. Epub 2013 Aug 2. Curr Microbiol. 2013. PMID: 23907491 Free PMC article.
References
-
- Abu Kwaik, Y., L. Y. Gao, O. S. Harb, and B. J. Stone. 1997. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol. Microbiol. 24:629-642. - PubMed
-
- Alli, O. A., S. Zink, N. K. Von Lackum, and Y. Abu-Kwaik. 2003. Comparative assessment of virulence traits in Legionella spp. Microbiology 149:631-641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

