The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli
- PMID: 15155671
- PMCID: PMC415714
- DOI: 10.1128/IAI.72.6.3609-3621.2004
The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli
Abstract
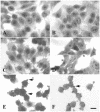
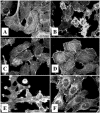
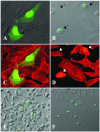
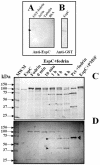
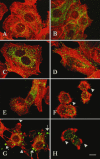
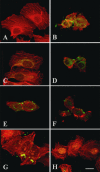
EspC (Escherichia coli secreted protein C) of enteropathogenic E. coli (EPEC) shows the three classical domains of the autotransporter proteins and has a conserved serine protease motif belonging to the SPATE (serine protease autotransporters of Enterobacteriaceae) subfamily. EspC and its homolog Pet in enteroaggregative E. coli (EAEC) bear the same sequence within the serine protease motif, and both proteins produce enterotoxic effects, suggesting that like Pet, EspC could be internalized to reach and cleave the calmodulin-binding domain of fodrin, causing actin cytoskeleton disruption. Even though both proteins cause cytoskeleton damage by virtue of their serine protease motifs, the following evidence supports the hypothesis that the mechanisms are different. (i) To obtain similar cytotoxic and cytoskeletal effects, a threefold-higher EspC concentration and a twofold-higher exposure time are needed. (ii) EspC internalization into epithelial cells takes more time (6 h) than Pet internalization (30 min), and the distributions of the two proteins inside the cells are also different. (iii) Both proteins have affinity for fodrin and cleave it, but the cleavage sites are different; EspC produces two cleavages, while Pet produces just one. (iv) EspC does not cause fodrin redistribution within epithelial cells. (v) An EspC serine protease motif mutant, but not a Pet serine protease mutant, competes with EspC by blocking cytoskeletal damage. All these data suggest that the protein conformational structure is very important for the activity of the catalytic site, influencing its interaction with the target protein and its internalization. The differences between these proteins may explain the reduced ability of EspC to cause cytopathic effects. However, these differences may confer a specialized role on EspC in the pathogenesis of EPEC, which is different from that of Pet in EAEC pathogenesis.
Figures


Similar articles
-
Curcumin Blocks Cytotoxicity of Enteroaggregative and Enteropathogenic Escherichia coli by Blocking Pet and EspC Proteolytic Release From Bacterial Outer Membrane.Front Cell Infect Microbiol. 2019 Sep 25;9:334. doi: 10.3389/fcimb.2019.00334. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31681620 Free PMC article.
-
Fodrin CaM-binding domain cleavage by Pet from enteroaggregative Escherichia coli leads to actin cytoskeletal disruption.Mol Microbiol. 2003 May;48(4):947-58. doi: 10.1046/j.1365-2958.2003.03492.x. Mol Microbiol. 2003. PMID: 12753188
-
Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption.Infect Immun. 2000 Oct;68(10):5920-7. doi: 10.1128/IAI.68.10.5920-5927.2000. Infect Immun. 2000. PMID: 10992503 Free PMC article.
-
Enteroaggregative Escherichia coli plasmid-encoded toxin.Future Microbiol. 2010 Jul;5(7):1005-13. doi: 10.2217/fmb.10.69. Future Microbiol. 2010. PMID: 20632801 Review.
-
Autotransporters and virulence of enteroaggregative E. coli.Gut Microbes. 2011 Jan-Feb;2(1):13-24. doi: 10.4161/gmic.2.1.14933. Gut Microbes. 2011. PMID: 21637014 Review.
Cited by
-
Efficient translocation of EspC into epithelial cells depends on enteropathogenic Escherichia coli and host cell contact.Infect Immun. 2006 Apr;74(4):2293-303. doi: 10.1128/IAI.74.4.2293-2303.2006. Infect Immun. 2006. PMID: 16552060 Free PMC article.
-
Blowing epithelial cell bubbles with GumB: ShlA-family pore-forming toxins induce blebbing and rapid cellular death in corneal epithelial cells.PLoS Pathog. 2019 Jun 20;15(6):e1007825. doi: 10.1371/journal.ppat.1007825. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31220184 Free PMC article.
-
Protease activity, secretion, cell entry, cytotoxicity, and cellular targets of secreted autotransporter toxin of uropathogenic Escherichia coli.Infect Immun. 2006 Nov;74(11):6124-34. doi: 10.1128/IAI.01086-06. Epub 2006 Sep 5. Infect Immun. 2006. PMID: 16954394 Free PMC article.
-
Prevalence, biogenesis, and functionality of the serine protease autotransporter EspP.Toxins (Basel). 2012 Dec 28;5(1):25-48. doi: 10.3390/toxins5010025. Toxins (Basel). 2012. PMID: 23274272 Free PMC article. Review.
-
Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence.Cell Mol Life Sci. 2014 Mar;71(5):745-70. doi: 10.1007/s00018-013-1355-8. Epub 2013 May 21. Cell Mol Life Sci. 2014. PMID: 23689588 Free PMC article. Review.
References
-
- Canizalez-Roman, A., and F. Navarro-Garcia. 2003. Fodrin CaM-binding domain cleavage by Pet from enteroaggregative Escherichia coli leads to actin cytoskeletal disruption. Mol. Microbiol. 48:947-958. - PubMed
-
- Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. - PubMed
-
- Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

