Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele
- PMID: 15155789
- PMCID: PMC1665015
- DOI: 10.1113/jphysiol.2004.062299
Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele
Abstract
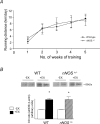
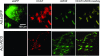
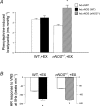
We tested the hypothesis that a single allele deletion of neuronal nitric oxide synthase (nNOS) would impair the neural control of heart rate following physical training, and that this phenotype could be restored following targeted gene transfer of nNOS. Voluntary wheel-running (+EX) in heterozygous nNOS knockout mice (nNOS(+/-), +EX; n= 52; peak performance 9.1 +/- 1.8 km day(-1)) was undertaken and compared to wild-type mice (n= 38; 9.5 +/- 0.8 km day(-1)). In anaesthetized wild-type mice, exercise increased phenylephrine-induced bradycardia by 67% (measured as heart rate change, in beats per minute, divided by the change in arterial blood pressure, in mmHg) or pulse interval response to phenylephrine by 52% (measured as interbeat interval change, in milliseconds, divided by the change in blood pressure). Heart rate changes or interbeat interval changes in response to right vagal nerve stimulation were also enhanced by exercise in wild-type atria (P < 0.05), whereas both in vivo and in vitro responses to exercise were absent in nNOS(+/-) mice. nNOS inhibition attenuated heart rate responses to vagal nerve stimulation in all atria (P < 0.05) and normalized the responses in wild-type, +EX with respect to wild-type with no exercise (-EX) atria. Atrial nNOS mRNA and protein were increased in wild-type, +EX compared to wild-type, -EX (P < 0.05), although exercise failed to have any effect in nNOS(+/-) atria. In vivo nNOS gene transfer using adenoviruses targeted to atrial ganglia enhanced choline acetyltransferase-nNOS co-localization (P < 0.05) and increased phenylephrine-induced bradycardia in vivo and heart rate responses to vagal nerve stimulation in vitro compared to gene transfer of enhanced green fluorescent protein (eGFP, P < 0.01). This difference was abolished by nNOS inhibition (P < 0.05). In conclusion, genomic regulation of NO bioavailability from nNOS in cardiac autonomic ganglia in response to training is dependent on both alleles of the gene. Although basal expression of nNOS is normal, polymorphisms of nNOS may interfere with neural regulation of heart rate following training. Targeted gene transfer of nNOS can restore this impairment.
Figures



References
-
- Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–3016. - PubMed
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Circulation. 2001;104:3097–3102. - PubMed
-
- Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training – what's the link? Exp Physiol. 2002;87:423–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

