Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis
- PMID: 15155879
- PMCID: PMC490037
- DOI: 10.1105/tpc.021568
Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis
Abstract
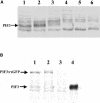
Light, in a quality- and quantity-dependent fashion, induces nuclear import of the plant photoreceptors phytochrome, promotes interaction of phytochrome A (phyA) and phyB with transcription factors including phytochrome interacting factor 3 (PIF3), and is thought to trigger a transcriptional cascade to regulate the expression of approximately 2500 genes in Arabidopsis thaliana. Here, we show that controlled degradation of the transcription factor PIF3 is a major regulatory step in light signaling. We demonstrate that accumulation of PIF3 in the nucleus in dark requires constitutive photomorphogenesis 1 (COP1), a negative regulator of photomorphogenesis, and show that red (R) and far-red light (FR) induce rapid degradation of the PIF3 protein. This process is controlled by the concerted action of the R/FR absorbing phyA, phyB, and phyD photoreceptors, and it is not affected by COP1. Rapid light-induced degradation of PIF3 indicates that interaction of PIF3 with these phytochrome species is transient. In addition, we provide evidence that the poc1 mutant, a postulated PIF3 overexpressor that displays hypersensitivity to R but not to FR, lacks detectable amounts of the PIF3 protein. Thus, we propose that PIF3 acts transiently, and its major function is to mediate phytochrome-induced signaling during the developmental switch from skotomorphogenesis to photomorphogenesis and/or dark to light transitions.
Copyright 2004 American Society of Plant Biologists
Figures
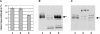







References
-
- Choi, G., Yi, H., Lee, J., Kwon, Y.K., Soh, M.S., Shin, B., Luka, Z., Hahn, T.R., and Song, P.S. (1999). Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401, 610–613. - PubMed
-
- Clack, T., Matthews, S., and Sharrock, R.A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequence and expression of PHYD and PHYE. Plant Mol. Biol. 25, 413–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

