Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice
- PMID: 15155903
- PMCID: PMC420410
- DOI: 10.1073/pnas.0400625101
Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice
Abstract
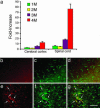
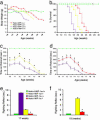
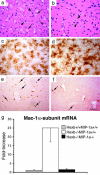
Sandhoff disease is a prototypical lysosomal storage disorder in which a heritable deficiency of a lysosomal enzyme, beta-hexosaminidase, results in the storage of the enzyme's substrates in lysosomes. As with many of the other lysosomal storage diseases, neurodegeneration is a prominent feature. Although the cellular and molecular pathways that underlie the neurodegenerative process are not yet fully understood, macrophage/microglial-mediated inflammation has been suggested as one possible mechanism. We now show that the expanded macrophage/microglial population in the CNS of Sandhoff disease mice is compounded by the infiltration of cells from the periphery. Coincident with the cellular infiltration was an increased expression of macrophage-inflammatory protein 1alpha (MIP-1alpha), a leukocyte chemokine, in astrocytes. Deletion of MIP-1alpha expression resulted in a substantial decrease in infiltration and macrophage/microglial-associated pathology together with neuronal apoptosis in Sandhoff disease mice. These mice without MIP-1alpha showed improved neurologic status and a longer lifespan. The results indicate that the pathogenesis of Sandhoff disease involves an increase in MIP-1alpha that induces monocytes to infiltrate the CNS, expand the activated macrophage/microglial population, and trigger apoptosis of neurons, resulting in a rapid neurodegenerative course.
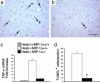
Figures

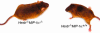
Similar articles
-
Specific induction of macrophage inflammatory protein 1-alpha in glial cells of Sandhoff disease model mice associated with accumulation of N-acetylhexosaminyl glycoconjugates.J Neurochem. 2005 Mar;92(6):1497-507. doi: 10.1111/j.1471-4159.2005.02986.x. J Neurochem. 2005. PMID: 15748167
-
Abnormal production of macrophage inflammatory protein-1alpha by microglial cell lines derived from neonatal brains of Sandhoff disease model mice.J Neurochem. 2009 Jun;109(5):1215-24. doi: 10.1111/j.1471-4159.2009.06041.x. Epub 2009 Mar 19. J Neurochem. 2009. PMID: 19302485
-
Metabolic correction in microglia derived from Sandhoff disease model mice.J Neurochem. 2005 Sep;94(6):1631-8. doi: 10.1111/j.1471-4159.2005.03317.x. Epub 2005 Aug 10. J Neurochem. 2005. PMID: 16092933
-
Macrophage inflammatory protein-1.Int J Biochem Cell Biol. 2004 Oct;36(10):1882-6. doi: 10.1016/j.biocel.2003.10.019. Int J Biochem Cell Biol. 2004. PMID: 15203102 Review.
-
[Sandhoff disease (beta-hexosaminidase alpha-chain deficiency)].Ryoikibetsu Shokogun Shirizu. 1998;(19 Pt 2):397-9. Ryoikibetsu Shokogun Shirizu. 1998. PMID: 9645091 Review. Japanese. No abstract available.
Cited by
-
Therapeutic approaches to the challenge of neuronal ceroid lipofuscinoses.Curr Pharm Biotechnol. 2011 Jun;12(6):867-83. doi: 10.2174/138920111795542633. Curr Pharm Biotechnol. 2011. PMID: 21235444 Free PMC article. Review.
-
Pre-clinical Mouse Models of Neurodegenerative Lysosomal Storage Diseases.Front Mol Biosci. 2020 Apr 15;7:57. doi: 10.3389/fmolb.2020.00057. eCollection 2020. Front Mol Biosci. 2020. PMID: 32351971 Free PMC article. Review.
-
The role of microglia in human disease: therapeutic tool or target?Acta Neuropathol. 2014 Sep;128(3):363-80. doi: 10.1007/s00401-014-1330-y. Epub 2014 Aug 9. Acta Neuropathol. 2014. PMID: 25107477 Free PMC article. Review.
-
Importance of macrophage inflammatory protein-1α and splenic macrophages in neurodegeneration induced by PVC-211 murine leukemia virus.Virology. 2011 Jan 20;409(2):198-203. doi: 10.1016/j.virol.2010.10.012. Epub 2010 Nov 4. Virology. 2011. PMID: 21051067 Free PMC article.
-
Biochemical evidence for superior correction of neuronal storage by chemically modified enzyme in murine mucopolysaccharidosis VII.Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):17022-7. doi: 10.1073/pnas.1214779109. Epub 2012 Oct 1. Proc Natl Acad Sci U S A. 2012. PMID: 23027951 Free PMC article.
References
-
- Gravel, R. A., Kabak, M. M., Proia, R. L., Sandhoff, K., Suzuki, K. & Suzuki, K. (2001) in The Metabolic and Molecular Basis of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Valle, D., Sly, W. S., Childs, B., Kinzler, K. W. & Vogelstein, B. (McGraw–Hill, New York), Vol. 3, pp. 3827-3876.
-
- Kolter, T., Proia, R. L. & Sandhoff, K. (2002) J. Biol. Chem. 277, 25859-25862. - PubMed
-
- Neufeld, E. F. (1991) Annu. Rev. Biochem. 60, 257-280. - PubMed
-
- Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (2001) in The Metabolic and Molecular Basis of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Valle, D., Sly, W. S., Childs, B., Kinzler, K. W. & Vogelstein, B. (McGraw-Hill, New York), pp. 3371-3896.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

