A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia
- PMID: 15159416
- PMCID: PMC2172350
- DOI: 10.1083/jcb.200401136
A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia
Abstract
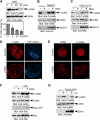
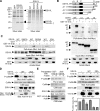
Cofilin mediates lamellipodium extension and polarized cell migration by stimulating actin filament dynamics at the leading edge of migrating cells. Cofilin is inactivated by phosphorylation at Ser-3 and reactivated by cofilin-phosphatase Slingshot-1L (SSH1L). Little is known of signaling mechanisms of cofilin activation and how this activation is spatially regulated. Here, we show that cofilin-phosphatase activity of SSH1L increases approximately 10-fold by association with actin filaments, which indicates that actin assembly at the leading edge per se triggers local activation of SSH1L and thereby stimulates cofilin-mediated actin turnover in lamellipodia. We also provide evidence that 14-3-3 proteins inhibit SSH1L activity, dependent on the phosphorylation of Ser-937 and Ser-978 of SSH1L. Stimulation of cells with neuregulin-1beta induced Ser-978 dephosphorylation, translocation of SSH1L onto F-actin-rich lamellipodia, and cofilin dephosphorylation. These findings suggest that SSH1L is locally activated by translocation to and association with F-actin in lamellipodia in response to neuregulin-1beta and 14-3-3 proteins negatively regulate SSH1L activity by sequestering it in the cytoplasm.
Figures



Similar articles
-
Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration.J Cell Biol. 2005 Oct 24;171(2):349-59. doi: 10.1083/jcb.200504029. Epub 2005 Oct 17. J Cell Biol. 2005. PMID: 16230460 Free PMC article.
-
Dual regulation of cofilin activity by LIM kinase and Slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells.Circ Res. 2008 Feb 29;102(4):432-8. doi: 10.1161/CIRCRESAHA.107.158923. Epub 2007 Dec 20. Circ Res. 2008. PMID: 18096821
-
Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension.Cell Motil Cytoskeleton. 1998;39(2):172-90. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. Cell Motil Cytoskeleton. 1998. PMID: 9484959
-
Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments.Biochemistry. 2003 Nov 25;42(46):13363-70. doi: 10.1021/bi034600x. Biochemistry. 2003. PMID: 14621980 Review.
-
Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation.Cell Signal. 2013 Feb;25(2):457-69. doi: 10.1016/j.cellsig.2012.11.001. Epub 2012 Nov 12. Cell Signal. 2013. PMID: 23153585 Review.
Cited by
-
Activation of ADF/cofilin by phosphorylation-regulated Slingshot phosphatase is required for the meiotic spindle assembly in Xenopus laevis oocytes.Mol Biol Cell. 2013 Jun;24(12):1933-46. doi: 10.1091/mbc.E12-12-0851. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615437 Free PMC article.
-
The role of slingshot-1L (SSH1L) in the differentiation of human bone marrow mesenchymal stem cells into cardiomyocyte-like cells.Molecules. 2012 Dec 17;17(12):14975-94. doi: 10.3390/molecules171214975. Molecules. 2012. PMID: 23247370 Free PMC article.
-
Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing.J Cell Biol. 2006 Dec 18;175(6):947-55. doi: 10.1083/jcb.200604176. J Cell Biol. 2006. PMID: 17178911 Free PMC article.
-
Cofilin and Actin Dynamics: Multiple Modes of Regulation and Their Impacts in Neuronal Development and Degeneration.Cells. 2021 Oct 12;10(10):2726. doi: 10.3390/cells10102726. Cells. 2021. PMID: 34685706 Free PMC article. Review.
-
Insulin receptor substrate-4 binds to Slingshot-1 phosphatase and promotes cofilin dephosphorylation.J Biol Chem. 2014 Sep 19;289(38):26302-26313. doi: 10.1074/jbc.M114.565945. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100728 Free PMC article.
References
-
- Arber, S., F.A. Barbayannis, H. Hanser, C. Schneider, C.A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393:805–809. - PubMed
-
- Chen, H., B.W. Bernstein, and J.R. Bamburg. 2000. Regulating actin-filament dynamics in vivo. Trends Biochem. Sci. 25:19–23. - PubMed
-
- Dawe, H.R., L.S. Minamide, J.R. Bamburg, and L.P. Cramer. 2003. ADF/Cofilin controls cell polarity during fibroblast migration. Curr. Biol. 13:252–257. - PubMed
-
- Gohla, A., and G.M. Bokoch. 2002. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 12:1704–1710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

