MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction
- PMID: 15159418
- PMCID: PMC2172359
- DOI: 10.1083/jcb.200307164
MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction
Abstract
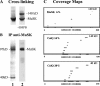
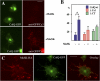
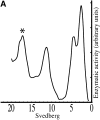
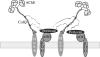
At the neuromuscular junction, acetylcholinesterase (AChE) is mainly present as asymmetric forms in which tetramers of catalytic subunits are associated to a specific collagen, collagen Q (ColQ). The accumulation of the enzyme in the synaptic basal lamina strictly relies on ColQ. This has been shown to be mediated by interaction between ColQ and perlecan, which itself binds dystroglycan. Here, using transfected mutants of ColQ in a ColQ-deficient muscle cell line or COS-7 cells, we report that ColQ clusterizes through a more complex mechanism. This process requires two heparin-binding sites contained in the collagen domain as well as the COOH terminus of ColQ. Cross-linking and immunoprecipitation experiments in Torpedo postsynaptic membranes together with transfection experiments with muscle-specific kinase (MuSK) constructs in MuSK-deficient myotubes or COS-7 cells provide the first evidence that ColQ binds MuSK. Together, our data suggest that a ternary complex containing ColQ, perlecan, and MuSK is required for AChE clustering and support the notion that MuSK dictates AChE synaptic localization at the neuromuscular junction.
Figures





References
-
- Anglister, L., J. Eichler, M. Szabo, B. Haesaert, and M.M. Salpeter. 1998. 125I-labeled fasciculin 2: a new tool for quantitation of acetylcholinesterase densities at synaptic sites by EM-autoradiography. J. Neurosci. Methods. 81:63–71. - PubMed
-
- Arikawa-Hirasawa, E., S.G. Rossi, R.L. Rotundo, and Y. Yamada. 2002. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat. Neurosci. 5:119–123. - PubMed
-
- Bon, S., F. Coussen, and J. Massoulié. 1997. Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem. 272:3016–3021. - PubMed
-
- Burden, S.J., R.L. DePalma, and G.S. Gottesman. 1983. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 35:687–692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

