Protein kinase CK2 modulates developmental functions of the abscisic acid responsive protein Rab17 from maize
- PMID: 15159549
- PMCID: PMC470767
- DOI: 10.1073/pnas.0306154101
Protein kinase CK2 modulates developmental functions of the abscisic acid responsive protein Rab17 from maize
Abstract
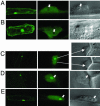
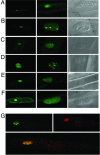
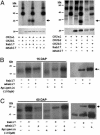
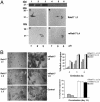
The maize abscisic acid responsive protein Rab17 is a highly phosphorylated late embryogenesis abundant protein involved in plant responses to stress. In this study, we provide evidence of the importance of Rab17 phosphorylation by protein kinase CK2 in growth-related processes under stress conditions. We show the specific interaction of Rab17 with the CK2 regulatory subunits CK2 beta-1 and CK2 beta-3, and that these interactions do not depend on the phosphorylation state of Rab17. Live-cell fluorescence imaging of both CK2 and Rab17 indicates that the intracellular dynamics of Rab17 are regulated by CK2 phosphorylation. We found both CK2 beta subunits and Rab17 distributed over the cytoplasm and nucleus. By contrast, catalytic CK2 alpha subunits and a Rab17 mutant protein (mRab17) that is not a substrate for CK2 phosphorylation remain accumulated in the nucleoli. A dual-color image shows that the CK2 holoenzyme accumulates mainly in the nucleus. The importance of Rab17 phosphorylation in vivo was assessed in transgenic plants. The overexpression of Rab17, but not mRab17, arrests the process of seed germination under osmotic stress conditions. Thus, the role of Rab17 in growth processes is mediated through its phosphorylation by protein kinase CK2.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

