Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor
- PMID: 15161637
- PMCID: PMC1615783
- DOI: 10.1016/S0002-9440(10)63761-3
Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor
Abstract
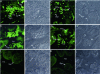
In pregnant women infected with Plasmodium falciparum, the parasite-infected red blood cells (IRBCs) sequester in the placenta through chondroitin 4-sulfate (C4S)-mediated adherence. The pattern of IRBC adherence in P. falciparum-infected placenta has been controversial. Moreover, the identity of the chondroitin sulfate proteoglycan (CSPG) receptor, that mediates IRBC adherence, and its location in the placenta have not been established. This study, using immunohistochemical techniques, clearly shows, for the first time, that the low-sulfated CSPGs of the placenta are localized predominantly in the intervillous space. Ex vivo IRBC adherence analyses demonstrate that the IRBCs are adhered to the CSPG receptors in the placenta in a C4S-dependent manner. This IRBC binding pattern was similar to that observed in P. falciparum-infected placentas. These data and the results of dual-fluorescence staining of the endogenous RBCs and syncytiotrophoblasts, and co-localization of CSPG and IRBC adherence unequivocally establish that the low-sulfated CSPGs are the major natural receptors for IRBC adherence in the placenta. Further, it was found that IRBCs adhere mainly in the intervillous space and also at significant levels to the syncytiotrophoblasts. Finally, the ex vivo IRBC adherence method described herein provides a reliable procedure for future studies for the assessment of the efficacy of C4S inhibitors and adhesion inhibitory antibodies.
Figures





References
-
- Trigg T, Kondrachine AV. The current global malaria situation. Sherman IW, editor. Washington DC: ASM Press,; Malaria—Parasite Biology, Pathogenesis, and Protection. 1998:pp 11–22.
-
- Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. - PubMed
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. - PubMed
-
- Pasloske BL, Howard RJ. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

