Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy
- PMID: 15161643
- PMCID: PMC1615769
- DOI: 10.1016/S0002-9440(10)63767-4
Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy
Abstract
This study was performed to quantitate and characterize the mononuclear phagocytes (MPs) in human immunodeficiency virus encephalopathy (HIVE) by immunohistochemistry in an effort to gain insights into potential mechanisms of central nervous system (CNS) accumulation. Single- and double-labeled studies using antibodies against CD14, CD16, CD68, proliferating cell nuclear antigen (PCNA), Ki-67, von Willebrand factor, and HIV-1 p24 were performed using brain tissue from patients with HIVE, HIV-1 infection without encephalitis, and seronegative controls. A substantial increase in MPs was observed in CNS tissue from patients with HIVE, relative to seronegative controls and patients with acquired immune deficiency syndrome but without encephalitis, as determined by CD68 and CD16 immunohistochemistry. A large proportion of CD16+ MPs in HIVE CNS tissue were PCNA+, but do not appear to be proliferating, based on limited Ki-67 positivity. Although virtually all cells positive for HIV-1 p24 were PCNA+, there were many PCNA+ cells where HIV-1 p24 expression was not detected. PCNA positivity was also observed in some endothelial cells and ependymal cells in HIVE CNS. Our results would support a role for HIV-1-induced alterations in MP trafficking and homeostasis in the pathogenesis of HIVE.
Figures


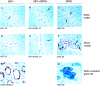


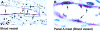
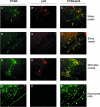
References
-
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. - PubMed
-
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, Visscher BR, Concha M, Saah S. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. - PubMed
-
- Rostad SW, Sumi SM, Shaw CM, Olson K, McDougall JK. Human immunodeficiency virus (HIV) infection in brains with AIDS-related leukoencephalopathy. AIDS Res Hum Retroviruses. 1987;3:363–373. - PubMed
-
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

