Use of neoadjuvant chemotherapy prior to radical hysterectomy in cervical cancer: monitoring tumour shrinkage and molecular profile on magnetic resonance and assessment of 3-year outcome
- PMID: 15162152
- PMCID: PMC2409522
- DOI: 10.1038/sj.bjc.6601870
Use of neoadjuvant chemotherapy prior to radical hysterectomy in cervical cancer: monitoring tumour shrinkage and molecular profile on magnetic resonance and assessment of 3-year outcome
Abstract
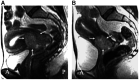
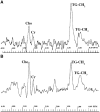
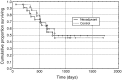
The objective of this study is to assess tumour response to neoadjuvant chemotherapy prior to radical hysterectomy in cervical cancer using magnetic resonance (MR) to monitor tumour volume and changes in molecular profile and to compare the survival to that of a control group. Eligibility included Stage Ib-IIb previously untreated cervical tumours >10 cm(3). Neoadjuvant chemotherapy in 22 patients (methotrexate 300 mg x m(-2) (with folinic acid rescue), bleomycin 30 mg x m(-2), cisplatin 60 mg m(-2)) was repeated twice weekly for three courses and followed by radical hysterectomy. Post-operative radiotherapy was given in 14 cases. A total of 23 patients treated either with radical surgery or chemoradiotherapy over the same time period comprised the nonrandomised control group. MR scans before and after neoadjuvant chemotherapy and in the control group documented tumour volume on imaging and metabolites on in vivo spectroscopy. Changes were compared using a paired t-test. Survival was calculated using the Kaplan-Meier method. There were no significant differences between the neoadjuvant chemotherapy and control groups in age (mean, s.d. 43.3+/-10, 44.7+/-8.5 years, respectively, P=0.63) or tumour volume (medians, quartiles 35.8, 17.8, 57.7 cm(3) vs 23.0, 15.0, 37.0 cm(3), respectively, P=0.068). The reduction in tumour volume post-chemotherapy (median, quartiles 7.5, 3.0, 19.0 cm(3)) was significant (P=0.002). The reduction in -CH(2) triglyceride approached significance (P=0.05), but other metabolites were unchanged. The 3-year survival in the chemotherapy group (49.1%) was not significantly different from the control group (46%, P=0.94). There is a significant reduction in tumour volume and -CH(2) triglyceride levels after neoadjuvant chemotherapy, but there is no survival advantage.
Figures


References
-
- Aoki Y, Tomita M, Sato T, Watanabe M, Kase H, Fujita K, Kurata H, Tanaka K (2001) Neoadjuvant chemotherapy for patients younger than 50 y with high-risk squamous cell carcinoma of the cervix. Gynecol Oncol 83: 263–267 - PubMed
-
- Benedetti-Panici P, Greggi S, Scambia G, Amoroso M, Salerno MG, Maneschi F, Cutillo G, Paratore MP, Scorpiglione N, Mancuso S (1998) Long-term survival following neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Eur J Cancer 34: 341–346 - PubMed
-
- Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F, Zola P, Mangioni C, Landoni F (2002) Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multi-center randomized trial. J Clin Oncol 20: 179–188 - PubMed
-
- Benedetti-Panici P, Scambia G, Baiocchi G, Gregi S, Ragusa G, Gallo A, Conte M, Battaglia F, Laurelli G, Rabitti C, Capelli A, Mancuso S (1991) Neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer: prognostic factors for response and survival. Cancer 67: 372–379 - PubMed
-
- Blankenberg FG, Katsikis PD, Storrs RW, Beaulieu C, Spielman D, Chen JY, Naumovski L, Tait JF (1997) Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood 89: 3778–3786 - PubMed

