Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues
- PMID: 15163627
- PMCID: PMC2775443
- DOI: 10.1242/dev.01158
Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues
Abstract
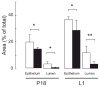
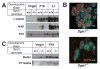
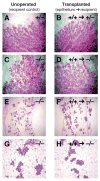
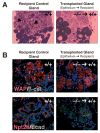
Mammary gland development is a complex process that is dependent on interactions between the developing mammary epithelium and the surrounding stromal tissues. We show that mice lacking the triglyceride synthesis enzyme acyl CoA:diacylglycerol transferase 1 (DGAT1) have impaired mammary gland development, characterized by decreased epithelial proliferation and alveolar development, and reduced expression of markers of functional differentiation. Transplantation studies demonstrate that the impaired development results from a deficiency of DGAT1 in both the stromal and epithelial tissues. Our findings are the first to link defects in stromal lipid metabolism to impaired mammary gland development.
Figures





References
-
- Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem. 1980;49:459–487. - PubMed
-
- Brindley DN. Metabolism of triacylglycerols. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier; 1991. pp. 171–203.
-
- Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210:96–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

