Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein
- PMID: 15163699
- PMCID: PMC6729370
- DOI: 10.1523/JNEUROSCI.5400-03.2004
Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein
Abstract
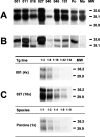
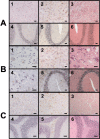
The bovine-porcine species barrier to bovine spongiform encephalopathy (BSE) infection was explored by generating transgenic mouse lines expressing the porcine prion protein (PrP) gene. All of the porcine transgenic (poTg) mice showed clinical signs of BSE after intracerebral inoculation with a high-titer BSE inoculum. The protease-resistant PrP (PrP(res)) was detected in 14% (3 of 22) of the BSE-infected poTg mice by immunohistochemical or immunoblot analysis. Despite being able to infect 42% (5 of 12) of control mice, a low-dose BSE inoculum failed to penetrate the species barrier in our poTg mouse model. The findings of these infectivity studies suggest that there is a strong species barrier between cows and pigs. However, after second-passage infection of poTg mice using brain homogenates of BSE-inoculated mice scoring negative for the incoming prion protein as inoculum, it was possible to detect the presence of the infectious agent. Thus, porcine-adapted BSE inocula were efficient at infecting poTg mice, giving rise to an incubation period substantially reduced from 300 to 177 d after inoculation and to the presence of PrP(res) in 100% (21 of 21) of the mice. We were therefore able to conclude that initial exposure to the bovine prion may lead to subclinical infection such that brain homogenates from poTg mice classified as uninfected on the basis of the absence of PrP(res) are infectious when used to reinoculate poTg mice. Collectively, our findings suggest that these poTg mice could be used as a sensitive bioassay model for prion detection in pigs.
Figures



References
-
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL (1996) A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal 13: 159-163. - PubMed
-
- Brun A, Castilla J, Ramírez MA, Prager K, Parra B, Salguero FJ, Shiveral D, Sánchez C, Sánchez-Vizcaíno JM, Douglas A, Torres JM (2004) Proteinase K enhanced immunorreactivity of the prion protein specific monoclonal antibody 2A11. J Neurosci Res 48: 75-83. - PubMed
-
- Castilla J, Gutiérrez Adán A, Brun A, Pintado B, Ramírez MA, Parra B, Doyle D, Rogers M, Salguero J, Sánchez C, Sánchez-Vizcaíno JM, Torres JM (2003) Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol 148: 677-691. - PubMed
-
- Collinge J, Rossor M (1996) A new variant of prion disease. Lancet 347: 916-917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
