Inhibition of reovirus by mycophenolic acid is associated with the M1 genome segment
- PMID: 15163710
- PMCID: PMC416527
- DOI: 10.1128/JVI.78.12.6171-6179.2004
Inhibition of reovirus by mycophenolic acid is associated with the M1 genome segment
Abstract
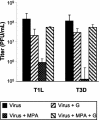
Mycophenolic acid (MPA), an inhibitor of IMP dehydrogenase, inhibits reovirus replication and viral RNA and protein production. In mouse L929 cells, antiviral effects were greatest at 30 microg of MPA/ml. At this dosage, MPA inhibited replication of reovirus strain T3D more than 1,000-fold and inhibited replication of reovirus strain T1L nearly 100-fold, compared to non-drug-treated controls. Genetic reassortant analysis indicated the primary determinant of strain-specific differences in sensitivity to MPA mapped to the viral M1 genome segment, which encodes the minor core protein mu2. MPA also inhibited replication of both strains of reovirus in a variety of other cell lines, including Vero monkey kidney and U373 human astrocytoma cells. Addition of exogenous guanosine to MPA-treated reovirus-infected cells restored viral replicative capacity to nearly normal levels. These results suggest the mu2 protein is involved in the uptake and processing of GTP in viral transcription in infected cells and strengthens the evidence that the mu2 protein can function as an NTPase and is likely a transcriptase cofactor.
Figures






References
-
- Allison, A. C., and E. M. Eugui. 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47:85-118. - PubMed
-
- Behrend, M., R. Lueck, and R. Pichlmayr. 1997. Long-term experience with mycofenolate mofetil in the prevention of renal allograft rejection. Transplant. Proc. 29:2927-2929. - PubMed
-
- Brown, E. G., M. L. Nibert, and B. N. Fields. 1983. The L2 gene of reovirus serotype 3 controls the capacity to interfere, accumulate deletions, and establish persistent infection, p. 275-287. In R. W. Compans and D. H. L. Bishop (ed.), Double-stranded RNA viruses. Elsevier Biomedical, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

