PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes
- PMID: 15163724
- PMCID: PMC416516
- DOI: 10.1128/JVI.78.12.6304-6312.2004
PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes
Abstract
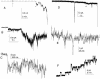
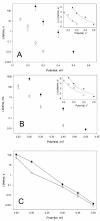
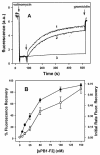
A frameshifted region of the influenza A virus PB1 gene encodes a novel protein, termed PB1-F2, a mitochondrial protein that can induce cell death. Many proapoptotic proteins are believed to act at the mitochondrial outer membrane to form an apoptotic pore with lipids. We studied the interaction of isolated, synthetic PB1-F2 (sPB1-F2) peptide with planar phospholipid bilayer membranes. The presence of nanomolar concentrations of peptide in the bathing solution induced a transmembrane conductance that increased in a potential-dependent manner. Positive potential on the side of protein addition resulted in a severalfold increase in the rate of change of membrane conductance. sPB1-F2-treated membranes became permeable to monovalent cations, chloride, and to a lesser extent, divalent ions. Despite various experimental conditions, we did not detect the distinctive conductance levels typical of large, stable pores, protein channels, or even pores that are partially proteinaceous. Rather, membrane conductance induced by sPB1-F2 fluctuated and visited almost all conductance values. sPB1-F2 also dramatically decreased bilayer stability in an electric field, consistent with a decrease in the line tension of a lipidic pore. Since similar membrane-destabilizing profiles are seen with proapoptotic proteins (e.g., Bax) and the cytoplasmic helix of human immunodeficiency virus gp41, we suggest that the basis for sPB1-F2-induced cell death may be the permeabilization and destabilization of mitochondrial membranes, leading to macromolecular leakage and apoptosis.
Figures


References
-
- Abidor, I. G., V. B. Arakelyan, L. V. Chernomordik, Y. A. Chizmadzhev, V. F. Pastushenko, and M. R. Tarasevich. 1979. Electrical breakdown of BLM: main experimental facts and their qualitative discussion. Bioelectrochem. Bioenerget. 6:37-52.
-
- Barlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. - PubMed
-
- Basañez, G., A. Nechushtan, O. Drozhinin, A. Chanturiya, E. Choe, S. Tutt, K. A. Wood, Y.-T. Hsu, J. Zimmerberg, and R. J. Youle. 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA 96:5492-5497. - PMC - PubMed
-
- Basañez, G., J. C. Sharpe, J. Galanis, T. B. Brandt, J. M. Hardwick, and J. Zimmerberg. 2002. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277:49360-49365. - PubMed
-
- Basañez, G., J. Zhang, B. N. Chau, G. I. Maksaev, V. A. Frolov, T. A. Brandt, J. Burch, J. M. Hardwick, and J. Zimmerberg. 2001. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J. Biol. Chem. 276:31083-31091. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

