Lyssavirus matrix protein induces apoptosis by a TRAIL-dependent mechanism involving caspase-8 activation
- PMID: 15163747
- PMCID: PMC416538
- DOI: 10.1128/JVI.78.12.6543-6555.2004
Lyssavirus matrix protein induces apoptosis by a TRAIL-dependent mechanism involving caspase-8 activation
Abstract
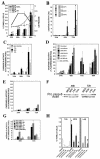
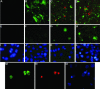
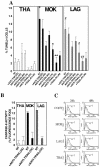
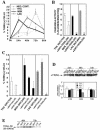
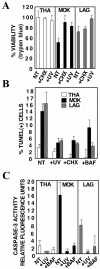
Lyssaviruses, which are members of the Rhabdoviridae family, induce apoptosis, which plays an important role in the neuropathogenesis of rabies. However, the mechanisms by which these viruses mediate neuronal apoptosis have not been elucidated. Here we demonstrate that the early induction of apoptosis in a model of lyssavirus-infected neuroblastoma cells involves a TRAIL-dependent pathway requiring the activation of caspase-8 but not of caspase-9 or caspase-10. The activation of caspase-8 results in the activation of caspase-3 and caspase-6, as shown by an increase in the cleavage of the specific caspase substrate in lyssavirus-infected cells. However, neither caspase-1 nor caspase-2 activity was detected during the early phase of infection. Lyssavirus-mediated cell death involves an interaction between TRAIL receptors and TRAIL, as demonstrated by experiments using neutralizing antibodies and soluble decoy TRAIL-R1/R2 receptors. We also demonstrated that the decapsidation and replication of lyssavirus are essential for inducing apoptosis, as supported by UV inactivation, cycloheximide treatment, and the use of bafilomycin A1 to inhibit endosomal acidification. Transfection of cells with the matrix protein induced apoptosis using pathways similar to those described in the context of viral infection. Furthermore, our data suggest that the matrix protein of lyssaviruses plays a major role in the early induction of TRAIL-mediated apoptosis by the release of a soluble, active form of TRAIL. In our model, Fas ligand (CD95L) appears to play a limited role in lyssavirus-mediated neuroblastoma cell death. Similarly, tumor necrosis factor alpha does not appear to play an important role.
Figures
Similar articles
-
The monoclonal antibody 225 activates caspase-8 and induces apoptosis through a tumor necrosis factor receptor family-independent pathway.Oncogene. 2001 Jun 21;20(28):3726-34. doi: 10.1038/sj.onc.1204490. Oncogene. 2001. PMID: 11439335
-
Drug-mediated sensitization to TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma cells proceeds via activation of intrinsic and extrinsic pathways and caspase-dependent cleavage of XIAP, Bcl-xL and RIP.Oncogene. 2004 Jul 15;23(32):5415-25. doi: 10.1038/sj.onc.1207704. Oncogene. 2004. PMID: 15094781
-
Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cells.Cancer Res. 2000 Dec 15;60(24):7149-55. Cancer Res. 2000. PMID: 11156424
-
Targeting the tumor necrosis factor-related apoptosis-inducing ligand path in neuroblastoma.Cancer Lett. 2003 Jul 18;197(1-2):137-43. doi: 10.1016/s0304-3835(03)00093-4. Cancer Lett. 2003. PMID: 12880973 Review.
-
Reovirus-induced apoptosis: A minireview.Apoptosis. 2003 Mar;8(2):141-50. doi: 10.1023/a:1022966508671. Apoptosis. 2003. PMID: 12766474 Review.
Cited by
-
Gene order rearrangement of the M gene in the rabies virus leads to slower replication.Virusdisease. 2014;25(3):365-71. doi: 10.1007/s13337-014-0220-1. Epub 2014 Jun 7. Virusdisease. 2014. PMID: 25674605 Free PMC article.
-
Regulation of NF-κB by the p105-ABIN2-TPL2 complex and RelAp43 during rabies virus infection.PLoS Pathog. 2017 Oct 30;13(10):e1006697. doi: 10.1371/journal.ppat.1006697. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29084252 Free PMC article.
-
Mechanisms of reovirus-induced cell death and tissue injury: role of apoptosis and virus-induced perturbation of host-cell signaling and transcription factor activation.Viral Immunol. 2005;18(1):89-115. doi: 10.1089/vim.2005.18.89. Viral Immunol. 2005. PMID: 15802955 Free PMC article. Review.
-
Subversion of the Immune Response by Rabies Virus.Viruses. 2016 Aug 19;8(8):231. doi: 10.3390/v8080231. Viruses. 2016. PMID: 27548204 Free PMC article. Review.
-
Evaluation of high-throughput sequencing for identifying known and unknown viruses in biological samples.J Clin Microbiol. 2011 Sep;49(9):3268-75. doi: 10.1128/JCM.00850-11. Epub 2011 Jun 29. J Clin Microbiol. 2011. PMID: 21715589 Free PMC article.
References
-
- Adle-Biassette, H., H. Bourhy, M. Gisselbrecht, F. Chretien, L. Wingertsmann, M. Baudrimont, Y. Rotivel, B. Godeau, and F. Gray. 1996. Rabies encephalitis in a patient with AIDS: a clinicopathological study. Acta Neuropathol. 92:415-420. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255-260. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. - PubMed
-
- Barker, V., G. Middleton, F. Davey, and A. M. Davies. 2001. TNF-α contributes to the death of NGF-dependent neurons during development. Nat. Neurosci. 4:1194-1198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

