Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus
- PMID: 15163751
- PMCID: PMC416546
- DOI: 10.1128/JVI.78.12.6595-6609.2004
Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus
Abstract
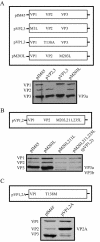
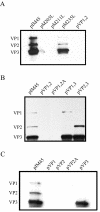
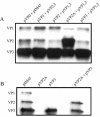
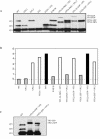
Direct insertion of amino acid sequences into the adeno-associated virus type 2 (AAV) capsid open reading frame (cap ORF) is one strategy currently being developed for retargeting this prototypical gene therapy vector. While this approach has successfully resulted in the formation of AAV particles that have expanded or retargeted viral tropism, the inserted sequences have been relatively short, linear receptor binding ligands. Since many receptor-ligand interactions involve nonlinear, conformation-dependent binding domains, we investigated the insertion of full-length peptides into the AAV cap ORF. To minimize disruption of critical VP3 structural domains, we confined the insertions to residue 138 within the VP1-VP2 overlap, which has been shown to be on the surface of the particle following insertion of smaller epitopes. The insertion of coding sequences for the 8-kDa chemokine binding domain of rat fractalkine (CX3CL1), the 18-kDa human hormone leptin, and the 30-kDa green fluorescent protein (GFP) after residue 138 failed to lead to formation of particles due to the loss of VP3 expression. To test the ability to complement these insertions with the missing capsid proteins in trans, we designed a system for producing AAV vectors in which expression of one capsid protein is isolated and combined with the remaining two capsid proteins expressed separately. Such an approach allows for genetic modification of a specific capsid protein across its entire coding sequence leaving the remaining capsid proteins unaffected. An examination of particle formation from the individual components of the system revealed that genome-containing particles formed as long as the VP3 capsid protein was present and demonstrated that the VP2 capsid protein is nonessential for viral infectivity. Viable particles composed of all three capsid proteins were obtained from the capsid complementation groups regardless of which capsid proteins were supplied separately in trans. Significant overexpression of VP2 resulted in the formation of particles with altered capsid protein stoichiometry. The key finding was that by using this system we successfully obtained nearly wild-type levels of recombinant AAV-like particles with large ligands inserted after residue 138 in VP1 and VP2 or in VP2 exclusively. While insertions at residue 138 in VP1 significantly decreased infectivity, insertions at residue 138 that were exclusively in VP2 had a minimal effect on viral assembly or infectivity. Finally, insertion of GFP into VP1 and VP2 resulted in a particle whose trafficking could be temporally monitored by using confocal microscopy. Thus, we have demonstrated a method that can be used to insert large (up to 30-kDa) peptide ligands into the AAV particle. This system allows greater flexibility than current approaches in genetically manipulating the composition of the AAV particle and, in particular, may allow vector retargeting to alternative receptors requiring interaction with full-length conformation-dependent peptide ligands.
Figures



References
-
- Buning, H., M. U. Ried, L. Perabo, F. M. Gerner, N. A. Huttner, J. Enssle, and M. Hallek. 2003. Receptor targeting of adeno-associated virus vectors. Gene Ther. 10:1142-1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

