Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity
- PMID: 15165230
- PMCID: PMC4428336
- DOI: 10.1111/j.1365-2958.2004.04063.x
Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity
Abstract
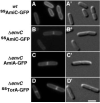
Bacterial cytokinesis is driven by the septal ring apparatus, the assembly of which in Escherichia coli is directed to mid-cell by the Min system. Despite suffering aberrant divisions at the poles, cells lacking the minCDE operon (Min(-)) have an almost normal growth rate. We developed a generally applicable screening method for synthetic lethality in E. coli, and used it to select for transposon mutations (slm) that are synthetically lethal (or sick) in combination with DeltaminCDE. One of the slm insertions mapped to envC (yibP), proposed to encode a lysostaphin-like, metallo-endopeptidase that is exported to the periplasm by the general secretory (Sec) pathway. Min(-) EnvC(-) cells showed a severe division defect, supporting a role for EnvC in septal ring function. Accordingly, we show that an EnvC-green fluorescent protein fusion, when directed to the periplasm via the twin-arginine export system, is both functional and part of the septal ring apparatus. Using an in-gel assay, we also present evidence that EnvC possesses murein hydrolytic activity. Our results suggest that EnvC plays a direct role in septal murein cleavage to allow outer membrane constriction and daughter cell separation. By uncovering genetic interactions, the synthetic lethal screen described here provides an attractive new tool for studying gene function in E. coli.
Figures







References
-
- Åkerlund T, Bernander R, Nordström K. Cell division in Escherichia coli minB mutants. Mol Microbiol. 1992;6:2073–2083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

