ACE2: from vasopeptidase to SARS virus receptor
- PMID: 15165741
- PMCID: PMC7119032
- DOI: 10.1016/j.tips.2004.04.001
ACE2: from vasopeptidase to SARS virus receptor
Abstract
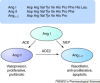
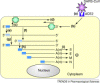
The zinc metallopeptidase angiotensin-converting enzyme 2 (ACE2) is the only known human homologue of the key regulator of blood pressure angiotensin-converting enzyme (ACE). Since its discovery in 2000, ACE2 has been implicated in heart function, hypertension and diabetes, with its effects being mediated, in part, through its ability to convert angiotensin II to angiotensin-(1-7). Unexpectedly, ACE2 also serves as the cellular entry point for the severe acute respiratory syndrome (SARS) virus and the enzyme is therefore a prime target for pharmacological intervention on several disease fronts.
Figures
References
-
- Turner A.J, Hooper N.M. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol. Sci. 2002;23:177–183. - PubMed
-
- Tipnis S.R. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. - PubMed
-
- Donoghue M. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. - PubMed
-
- Zisman L.S. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles. Evidence for up-regulation of the angiotensin-converting enzyme homologue ACE2. Circulation. 2003;108:1707–1712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

