Mouse models of childhood cancer of the nervous system
- PMID: 15166259
- PMCID: PMC1770320
- DOI: 10.1136/jcp.2003.009910
Mouse models of childhood cancer of the nervous system
Erratum in
- J Clin Pathol. 2004 Aug;57(8):896
Abstract
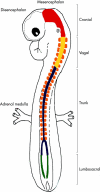
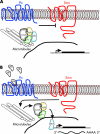
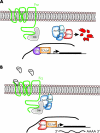
Targeted cancer treatments rely on understanding signalling cascades, genetic changes, and compensatory programmes activated during tumorigenesis. Increasingly, pathologists are required to interpret molecular profiles of tumour specimens to target new treatments. This is challenging because cancer is a heterogeneous disease-tumours change over time in individual patients and genetic lesions leading from preneoplasia to malignancy can differ substantially between patients. For childhood tumours of the nervous system, the challenge is even greater, because tumours arise from progenitor cells in a developmental context different from that of the adult, and the cells of origin, neural progenitor cells, show considerable temporal and spatial heterogeneity during development. Thus, the underlying mechanisms regulating normal development of the nervous system also need to be understood. Many important advances have come from model mouse genetic systems. This review will describe several mouse models of childhood tumours of the nervous system, emphasising how understanding the normal developmental processes, combined with mouse models of cancer and the molecular pathology of the human diseases, can provide the information needed to treat cancer more effectively.
Figures


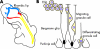

Similar articles
-
Medulloblastoma and retinoblastoma: oncology recapitulates ontogeny.Cell Cycle. 2004 Jul;3(7):917-9. Epub 2004 Jul 8. Cell Cycle. 2004. PMID: 15254429 Review.
-
The molecular and genetic basis of neurological tumours.Nat Rev Cancer. 2002 Aug;2(8):616-26. doi: 10.1038/nrc866. Nat Rev Cancer. 2002. PMID: 12154354 Review.
-
Transgenic mice as research tools in neurocarcinogenesis.J Neurovirol. 1998 Apr;4(2):159-74. doi: 10.3109/13550289809114516. J Neurovirol. 1998. PMID: 9584953
-
Neuron navigator 3 alterations in nervous system tumors associate with tumor malignancy grade and prognosis.Genes Chromosomes Cancer. 2013 Feb;52(2):191-201. doi: 10.1002/gcc.22019. Epub 2012 Oct 25. Genes Chromosomes Cancer. 2013. PMID: 23097141
-
Mouse models of human cancer consortium symposium on nervous system tumors.Cancer Res. 2003 Jun 1;63(11):3001-4. Cancer Res. 2003. PMID: 12782610
Cited by
-
MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development.Am J Pathol. 2009 Aug;175(2):856-66. doi: 10.2353/ajpath.2009.090019. Epub 2009 Jul 16. Am J Pathol. 2009. PMID: 19608868 Free PMC article.
-
Therapeutic targeting of both dihydroorotate dehydrogenase and nucleoside transport in MYCN-amplified neuroblastoma.Cell Death Dis. 2021 Aug 30;12(9):821. doi: 10.1038/s41419-021-04120-w. Cell Death Dis. 2021. PMID: 34462431 Free PMC article.
-
Residential Pesticide Exposures in Pregnancy and the Risk of Sporadic Retinoblastoma: A Report From the Children's Oncology Group.Am J Ophthalmol. 2017 Apr;176:166-173. doi: 10.1016/j.ajo.2017.01.016. Epub 2017 Jan 26. Am J Ophthalmol. 2017. PMID: 28131887 Free PMC article.
-
Metabolic profiling of the three neural derived embryonal pediatric tumors retinoblastoma, neuroblastoma and medulloblastoma, identifies distinct metabolic profiles.Oncotarget. 2018 Jan 11;9(13):11336-11351. doi: 10.18632/oncotarget.24168. eCollection 2018 Feb 16. Oncotarget. 2018. PMID: 29541417 Free PMC article.
-
Use of preclinical models to improve treatment of retinoblastoma.PLoS Med. 2005 Oct;2(10):e332. doi: 10.1371/journal.pmed.0020332. Epub 2005 Oct 25. PLoS Med. 2005. PMID: 16231976 Free PMC article. Review.
References
-
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer 2002;2:331–41. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003;3:203–16. - PubMed
-
- Basch ML, Selleck MA, Bronner-Fraser M, et al. Timing and competence of neural crest formation. Dev Neurosci 2000;22:217–27. - PubMed
-
- Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci 2001;2:333–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
