Utilizing tumor hypoxia to enhance oncolytic viral therapy in colorectal metastases
- PMID: 15166969
- PMCID: PMC1356298
- DOI: 10.1097/01.sla.0000128308.36393.38
Utilizing tumor hypoxia to enhance oncolytic viral therapy in colorectal metastases
Abstract
Objective: To determine the effects of hypoxia-induced ribonucleotide reductase (RR) production on herpes oncolytic viral therapy.
Summary background data: Hypoxia is a common tumor condition correlated with therapeutic resistance and metastases. Attenuated viruses offer a unique cancer treatment by specifically infecting and lysing tumor cells. G207 is an oncolytic herpes virus deficient in RR, a rate-limiting enzyme for viral replication.
Methods: A multimerized hypoxia-responsive enhancer was constructed (10xHRE) and functionally tested by luciferase assay. 10xHRE was cloned upstream of UL39, the gene encoding the large subunit of RR (10xHRE-UL39). CT26 murine colorectal cancer cells were transfected with 10xHRE-UL39, incubated in hypoxia (1% O2) or normoxia (21% O2), and infected with G207 for cytotoxicity assays. CT26 liver metastases, with or without 10xHRE-UL39, were created in syngeneic Balb/C mice (n = 40). Livers were treated with G207 or saline. Tumors were assessed and stained immunohistochemically for G207.
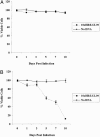
Results: 10xHRE increased luciferase expression 33-fold in hypoxia versus controls (P < 0.001). In normoxia, 10xHRE-UL39 transfection did not improve G207 cytotoxicity. In hypoxia, G207 cytotoxicity increased 87% with 10xHRE-UL39 transfection versus nontransfected cells (P < 0.001). CT26 were resistant to G207 alone. Combining 10xHRE-UL39 with G207 resulted in a 66% decrease in tumor weights (P < 0.0001) and a 65% reduction in tumor nodules (P < 0.0001) versus G207 monotherapy. 10xHRE-UL39-transfected tumors demonstrated greater viral staining.
Conclusions: Hypoxia-driven RR production significantly enhances viral cytotoxicity in vitro and reduces tumor burden in vivo. G207 combined with RR under hypoxic control is a promising treatment for colorectal cancer, which would otherwise be resistant to oncolytic herpes virus alone.
Figures





References
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. - PubMed
-
- Yuan J, Narayanan L, Rockwell S, et al. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. - PubMed
-
- Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. - PubMed
-
- Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

