Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing
- PMID: 15169849
- PMCID: PMC1664970
- DOI: 10.1113/jphysiol.2004.066001
Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing
Abstract
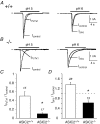
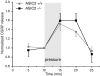
Mechanosensitive cation channels are thought to be crucial for different aspects of mechanoperception, such as hearing and touch sensation. In the nematode C. elegans, the degenerins MEC-4 and MEC-10 are involved in mechanosensation and were proposed to form mechanosensitive cation channels. Mammalian degenerin homologues, the H(+)-gated ASIC channels, are expressed in sensory neurones and are therefore interesting candidates for mammalian mechanosensors. We investigated the effect of an ASIC2 gene knockout in mice on hearing and on cutaneous mechanosensation and visceral mechanonociception. However, our data do not support a role of ASIC2 in those facets of mechanoperception.
Figures



References
-
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. - PubMed
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. - PubMed
-
- Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ J Biol Chem. 2000;275:28519–28525. - PubMed
-
- Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. - PubMed
-
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

