Role of pescadillo and upstream binding factor in the proliferation and differentiation of murine myeloid cells
- PMID: 15169904
- PMCID: PMC419857
- DOI: 10.1128/MCB.24.12.5421-5433.2004
Role of pescadillo and upstream binding factor in the proliferation and differentiation of murine myeloid cells
Abstract
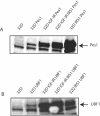
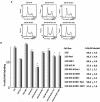
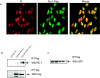
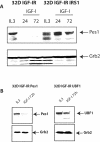
Pescadillo (PES1) and the upstream binding factor (UBF1) play a role in ribosome biogenesis, which regulates cell size, an important component of cell proliferation. We have investigated the effects of PES1 and UBF1 on the growth and differentiation of cell lines derived from 32D cells, an interleukin-3 (IL-3)-dependent murine myeloid cell line. Parental 32D cells and 32D IGF-IR cells (expressing increased levels of the type 1 insulin-like growth factor I [IGF-I] receptor [IGF-IR]) do not express insulin receptor substrate 1 (IRS-1) or IRS-2. 32D IGF-IR cells differentiate when the cells are shifted from IL-3 to IGF-I. Ectopic expression of IRS-1 inhibits differentiation and transforms 32D IGF-IR cells into a tumor-forming cell line. We found that PES1 and UBF1 increased cell size and/or altered the cell cycle distribution of 32D-derived cells but failed to make them IL-3 independent. PES1 and UBF1 also failed to inhibit the differentiation program initiated by the activation of the IGF-IR, which is blocked by IRS-1. 32D IGF-IR cells expressing PES1 or UBF1 differentiate into granulocytes like their parental cells. In contrast, PES1 and UBF1 can transform mouse embryo fibroblasts that have high levels of endogenous IRS-1 and are not prone to differentiation. Our results provide a model for one of the theories of myeloid leukemia, in which both a stimulus of proliferation and a block of differentiation are required for leukemia development.
Figures





Similar articles
-
Deletion of the pleckstrin and phosphotyrosine binding domains of insulin receptor substrate-2 does not impair its ability to regulate cell proliferation in myeloid cells.Endocrinology. 2004 Nov;145(11):5332-43. doi: 10.1210/en.2004-0668. Epub 2004 Aug 5. Endocrinology. 2004. PMID: 15297443
-
Dual regulation of upstream binding factor 1 levels by IRS-1 and ERKs in IGF-1-receptor signaling.J Cell Physiol. 2007 Sep;212(3):780-6. doi: 10.1002/jcp.21072. J Cell Physiol. 2007. PMID: 17443674
-
A mechanism for cell size regulation by the insulin and insulin-like growth factor-I receptors.Cancer Res. 2006 Dec 1;66(23):11106-9. doi: 10.1158/0008-5472.CAN-06-2641. Cancer Res. 2006. PMID: 17145851
-
Is cell size important?Cell Cycle. 2007 Apr 1;6(7):814-6. doi: 10.4161/cc.6.7.4049. Epub 2007 Apr 22. Cell Cycle. 2007. PMID: 17404503 Review.
-
Function of the IGF-I receptor in breast cancer.J Mammary Gland Biol Neoplasia. 2000 Jan;5(1):95-105. doi: 10.1023/a:1009523501499. J Mammary Gland Biol Neoplasia. 2000. PMID: 10791772 Review.
Cited by
-
The functional role of Pescadillo ribosomal biogenesis factor 1 in cancer.J Cancer. 2022 Jan 1;13(1):268-277. doi: 10.7150/jca.58982. eCollection 2022. J Cancer. 2022. PMID: 34976188 Free PMC article. Review.
-
Insulin receptor substrate (IRS)-1 regulates murine embryonic stem (mES) cells self-renewal.J Cell Physiol. 2007 Nov;213(2):445-53. doi: 10.1002/jcp.21185. J Cell Physiol. 2007. PMID: 17620314 Free PMC article.
-
TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways.Cell Signal. 2014 Dec;26(12):2773-81. doi: 10.1016/j.cellsig.2014.08.020. Epub 2014 Sep 2. Cell Signal. 2014. PMID: 25192910 Free PMC article.
-
Down-regulation of pescadillo inhibits proliferation and tumorigenicity of breast cancer cells.Cancer Sci. 2009 Dec;100(12):2255-60. doi: 10.1111/j.1349-7006.2009.01325.x. Epub 2009 Aug 25. Cancer Sci. 2009. PMID: 19764998 Free PMC article.
-
SUMOylation of PES1 upregulates its stability and function via inhibiting its ubiquitination.Oncotarget. 2016 Aug 2;7(31):50522-50534. doi: 10.18632/oncotarget.10494. Oncotarget. 2016. PMID: 27409667 Free PMC article.
References
-
- Arkins, S., N. Rebeiz, D. L. Brunke-Reese, C. Minshall, and K. W. Kelley. 1995. The colony-stimulating factors induce expression of insulin-like growth factor-I messenger ribonuclei acid during hematopoiesis. Endocrinology 136:1153-1160. - PubMed
-
- Belletti, B., M. Prisco, A. Morrione, B. Valentinis, M. Navarro, and R. Baserga. 2001. Regulation of Id2 gene expression by the IGF-I receptor requires signaling by phosphatidylinositol-3 kinase. J. Biol. Chem. 276:13867-13874. - PubMed
-
- Benezra, R., R. L. Davis, D. Lockshon, D. L. Turner, and H. Weintraub. 1990. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61:49-59. - PubMed
-
- Bohni, R., J. Riesco-Escovar, S. Oldham, W. Brogiolo, H. Stocker, B. F. Andruss, K. Beckingham, and E. Hafen. 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS-1. Cell 97:865-875. - PubMed
-
- Brown, G., M. T. Drayson, J. Durham, K. M. Toellner, P. J. Hughes, M. A. Choudhry, D. R. Taylor, and R. H. Michell. 2002. HL60 cells halted in G1 or S phase differentiate normally. Exp. Cell Res. 281:28-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
