Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway
- PMID: 15169906
- PMCID: PMC419880
- DOI: 10.1128/MCB.24.12.5447-5458.2004
Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway
Abstract
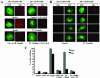
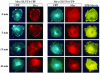
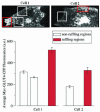
Glucose homeostasis is controlled in part by regulation of glucose uptake into muscle and adipose tissue. Intracellular membrane vesicles containing the GLUT4 glucose transporter move towards the cell cortex in response to insulin and then fuse with the plasma membrane. Here we show that the fusion step is retarded by the inhibition of phosphatidylinositol (PI) 3-kinase. Treatment of insulin-stimulated 3T3-L1 adipocytes with the PI 3-kinase inhibitor LY294002 causes the accumulation of GLUT4-containing vesicles just beneath the cell surface. This accumulation of GLUT4-containing vesicles near the plasma membrane prior to fusion requires an intact cytoskeletal network and the unconventional myosin motor Myo1c. Remarkably, enhanced Myo1c expression under these conditions causes extensive membrane ruffling and overrides the block in membrane fusion caused by LY294002, restoring the display of GLUT4 on the cell exterior. Ultrafast microscopic analysis revealed that insulin treatment leads to the mobilization of GLUT4-containing vesicles to these regions of Myo1c-induced membrane ruffles. Thus, localized membrane remodeling driven by the Myo1c motor appears to facilitate the fusion of exocytic GLUT4-containing vesicles with the adipocyte plasma membrane.
Figures



References
-
- Bennett, M. K., J. E. Garcia-Arraras, L. A. Elferink, K. Peterson, A. M. Fleming, C. D. Hazuka, and R. H. Scheller. 1993. The syntaxin family of vesicular transport receptors. Cell 74:863-873. - PubMed
-
- Borisy, G. G., and T. M. Svitkina. 2000. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12:104-112. - PubMed
-
- Bose, A., A. Guilherme, S. I. Robida, S. M. C. Nicoloro, Q. L. Zhou, Z. Y. Jiang, D. P. Pomerleau, and M. P. Czech. 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420:821-824. - PubMed
-
- Bretscher, M. S., and C. Aguado-Velasco. 1998. EGF induces recycling membrane to form ruffles. Curr. Biol. 8:721-724. - PubMed
-
- Broadie, K., A. Prokop, H. J. Bellen, C. J. O'Kane, K. L. Schulze, and S. T. Sweeney. 1995. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron 15:663-673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
