Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels
- PMID: 15172957
- PMCID: PMC1575051
- DOI: 10.1038/sj.bjp.0705841
Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels
Abstract
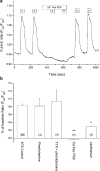
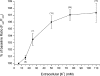
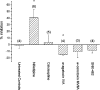
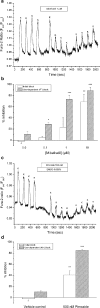
1. In this study, intracellular Ca(2+) was measured as the Fura-2 ratio (R) of fluorescence excited at 340 and 380 nm (F(340)/F(380)) in nonpressurized rat mesenteric small arterioles ( (lumen diameter) 10-25 microm). 2. The response to depolarization using 75 mm KCl was an increase in R from a baseline of 0.96+/-0.01 ([Ca(2+)](i) approximately 74 nm) to 1.04+/-0.01 ( approximately 128 nm) (n=80). The response to 75 mm K(+) was reversibly abolished in Ca(2+)-free physiological saline solution, whereas phentolamine (10 microm) or tetrodotoxin (1 microm) had no effects. LaCl(3) (200 microm) inhibited 61+/-9% of the response. 3. A [K(+)]-response curve indicated that the Ca(2+) response was activated between 15 and 25 mm K(+). The data suggest that the Ca(2+) response was caused by the activation of voltage-dependent Ca(2+) channels. 4. Mibefradil use dependently inhibited the Ca(2+) response to 75 mm K(+) by 29+/-2% (100 nm), 73+/-7% (1 microm) or 89+/-7% (10 microm). Pimozide (500 nm) use dependently inhibited the Ca(2+) response by 85+/-1%. 5. Nifedipine (1 microm) inhibited the Ca(2+) response to 75 mm K(+) by 41+/-12%. The response was not inhibited by calciseptine (500 nm), omega-agatoxin IVA (100 nm), omega-conotoxin MVIIA (500 nm), or SNX-482 (100 nm). 6. Using reverse transcriptase-polymerase chain reaction, it was shown that neither Ca(V)2.1a (P-type) nor Ca(V)2.1b (Q-type) voltage-dependent Ca(2+) channels were expressed in mesenteric arterioles, whereas the Ca(V)3.1 (T-type) channel was expressed. Furthermore, no amplification products were detected when using specific primers for the beta(1b), beta(2), or beta(3) auxiliary subunits of high-voltage-activated Ca(2+) channels. 7. The results suggest that the voltage-dependent Ca(2+) channel activated by sustained depolarization in mesenteric arterioles does not classify as any of the high-voltage-activated channels (L-, P/Q-, N-, or R-type), but is likely to be a T-type channel. The possibility that the sustained Ca(2+) influx observed was the result of a T-type window current is discussed.
Figures


Similar articles
-
Ca2+ entry via P/Q-type Ca2+ channels and the Na+/Ca2+ exchanger in rat and human neocortical synaptosomes.Naunyn Schmiedebergs Arch Pharmacol. 2002 Nov;366(5):458-63. doi: 10.1007/s00210-002-0629-8. Epub 2002 Sep 6. Naunyn Schmiedebergs Arch Pharmacol. 2002. PMID: 12382075
-
Involvement of different calcium channels in K+- and veratridine-induced increases of cytosolic calcium concentration in rat cerebral cortical synaptosomes.Naunyn Schmiedebergs Arch Pharmacol. 1997 Dec;356(6):797-805. doi: 10.1007/pl00005120. Naunyn Schmiedebergs Arch Pharmacol. 1997. PMID: 9453466
-
Calcium channels involved in K+- and veratridine-induced increase of cytosolic calcium concentration in human cerebral cortical synaptosomes.J Pharmacol Exp Ther. 1999 Sep;290(3):1126-31. J Pharmacol Exp Ther. 1999. PMID: 10454486
-
Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone.Am J Physiol. 1990 Jul;259(1 Pt 1):C3-18. doi: 10.1152/ajpcell.1990.259.1.C3. Am J Physiol. 1990. PMID: 2164782 Review.
-
Renal microcirculation and calcium channel subtypes.Curr Hypertens Rev. 2013 Aug;9(3):182-6. doi: 10.2174/1573402110666140131160617. Curr Hypertens Rev. 2013. PMID: 24479750 Free PMC article. Review.
Cited by
-
Interplay among distinct Ca2+ conductances drives Ca2+ sparks/spontaneous transient outward currents in rat cerebral arteries.J Physiol. 2017 Feb 15;595(4):1111-1126. doi: 10.1113/JP273329. Epub 2016 Dec 12. J Physiol. 2017. PMID: 27805790 Free PMC article.
-
Evidence both L-type and non-L-type voltage-dependent calcium channels contribute to cerebral artery vasospasm following loss of NO in the rat.Vascul Pharmacol. 2010 Sep-Oct;53(3-4):151-9. doi: 10.1016/j.vph.2010.06.002. Epub 2010 Jun 22. Vascul Pharmacol. 2010. PMID: 20601125 Free PMC article.
-
Calcium Channels in Vascular Smooth Muscle.Adv Pharmacol. 2017;78:49-87. doi: 10.1016/bs.apha.2016.08.002. Epub 2016 Oct 14. Adv Pharmacol. 2017. PMID: 28212803 Free PMC article. Review.
-
Voltage-dependent calcium channels of dog basilar artery.J Physiol. 2007 Apr 15;580(Pt. 2):523-41. doi: 10.1113/jphysiol.2006.126128. Epub 2006 Dec 21. J Physiol. 2007. PMID: 17185332 Free PMC article.
-
Vascular effects of calcium channel antagonists: new evidence.Drugs. 2005;65 Suppl 2:1-10. doi: 10.2165/00003495-200565002-00002. Drugs. 2005. PMID: 16398057 Review.
References
-
- ANDREASEN D., JENSEN B.L., HANSEN P.B., KWON T.H., NIELSEN S., SKØTT O. The α1G-subunit of a voltage-dependent Ca2+ channel is localized in rat distal nephron and collecting duct. Am. J. Physiol. Renal Physiol. 2000;279:F997–1005. - PubMed
-
- ARNOULT C., VILLAZ M., FLORMAN H.M. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol. Pharmacol. 1998;53:1104–1111. - PubMed
-
- BEEDLE A.M., HAMID J., ZAMPONI G.W. Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations. J. Membr. Biol. 2002;187:225–238. - PubMed
-
- BEZPROZVANNY I., TSIEN R.W. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol. Pharmacol. 1995;48:540–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

