Prostacyclin release and receptor activation: differential control of human pulmonary venous and arterial tone
- PMID: 15172959
- PMCID: PMC1575053
- DOI: 10.1038/sj.bjp.0705843
Prostacyclin release and receptor activation: differential control of human pulmonary venous and arterial tone
Abstract
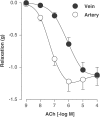
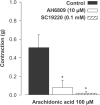
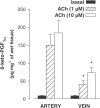
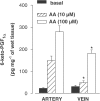
1. In human pulmonary vascular preparations, precontracted arteries were more sensitive to the relaxant effect of acetylcholine (ACh) than veins (pD(2) values: 7.25+/-0.08 (n=23) and 5.92+/-0.09 (n=25), respectively). Therefore, the role of prostacyclin (PGI(2)) was explored to examine whether this mediator may be responsible for the difference in relaxation. 2. In the presence of the cyclooxygenase (COX) inhibitor, indomethacin (INDO), the ACh relaxations were reduced in arteries but not in veins. On the contrary, an inhibitor (l-NOARG) of the nitric oxide synthase blocked preferentially the relaxation in veins. 3. A greater release of 6-keto-PGF(1alpha), the stable metabolite of PGI(2), was observed in arterial preparations than in venous preparations when stimulated with either ACh or arachidonic acid (AA). 4. Exogenous PGI(2) produced a reduced relaxant effect in the precontracted vein when compared with the artery. In the presence of the EP(1)-receptor antagonist AH6809, the PGI(2) relaxation of veins was similar to arteries. 5. In veins, AA (0.1 mm) produced a biphasic response, namely, a contraction peak (0.4-0.5 g) followed by a relaxation. These contractions in venous preparations were abolished either in the absence of endothelium or in the presence of INDO or an EP(1)-receptor antagonist (AH6809, SC19220). In the arterial preparations AA induced only relaxations. 6. In both vascular preparations, COX-1 but not the COX-2 protein was detected in microsomal preparations derived from homogenized tissues or freshly isolated endothelial cells. 7. The differential vasorelaxations induced by ACh may be explained, in part, by a more pronounced production and release of PGI(2) in human pulmonary arteries than in the veins. In addition, while PGI(2) induced relaxation by activation of IP-receptors in both types of vessels, a PGI(2) constrictor effect was responsible for masking the relaxation in the veins by activation of the EP(1)-receptor.
Figures





Similar articles
-
Prostanoid EP(1)- and TP-receptors involved in the contraction of human pulmonary veins.Br J Pharmacol. 2001 Dec;134(8):1671-8. doi: 10.1038/sj.bjp.0704423. Br J Pharmacol. 2001. PMID: 11739243 Free PMC article.
-
Concomitant activation of functionally opposing prostacyclin and thromboxane prostanoid receptors by cyclo-oxygenase-1-mediated prostacyclin synthesis in mouse arteries.Exp Physiol. 2012 Jul;97(7):895-904. doi: 10.1113/expphysiol.2011.063784. Epub 2012 Mar 23. Exp Physiol. 2012. PMID: 22447972
-
Prostanoid receptors involved in the relaxation of human pulmonary vessels.Br J Pharmacol. 1999 Feb;126(4):859-66. doi: 10.1038/sj.bjp.0702393. Br J Pharmacol. 1999. PMID: 10193765 Free PMC article.
-
Functional studies of leukotriene receptors in vascular tissues.Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 2):S107-11. doi: 10.1164/ajrccm.161.supplement_1.ltta-21. Am J Respir Crit Care Med. 2000. PMID: 10673237 Review.
-
Prostanoid action on the human pulmonary vascular system.Clin Exp Pharmacol Physiol. 1997 Dec;24(12):969-72. doi: 10.1111/j.1440-1681.1997.tb02730.x. Clin Exp Pharmacol Physiol. 1997. PMID: 9406667 Review.
Cited by
-
Levosimendan Relaxes Pulmonary Arteries and Veins in Precision-Cut Lung Slices - The Role of KATP-Channels, cAMP and cGMP.PLoS One. 2013 Jun 18;8(6):e66195. doi: 10.1371/journal.pone.0066195. Print 2013. PLoS One. 2013. PMID: 23824760 Free PMC article.
-
Cardiovascular agents affect the tone of pulmonary arteries and veins in precision-cut lung slices.PLoS One. 2011;6(12):e29698. doi: 10.1371/journal.pone.0029698. Epub 2011 Dec 27. PLoS One. 2011. PMID: 22216346 Free PMC article.
-
Nitric oxide deficiency promotes vascular side effects of cyclooxygenase inhibitors.Blood. 2006 Dec 15;108(13):4059-62. doi: 10.1182/blood-2006-02-005330. Epub 2006 Aug 24. Blood. 2006. PMID: 16931629 Free PMC article.
-
Characteristics of attenuated endothelium-dependent relaxation seen in rabbit intrapulmonary vein following chronic nitroglycerine administration.Br J Pharmacol. 2005 May;145(2):193-202. doi: 10.1038/sj.bjp.0706178. Br J Pharmacol. 2005. PMID: 15753949 Free PMC article.
-
PDGF-BB regulates the pulmonary vascular tone: impact of prostaglandins, calcium, MAPK- and PI3K/AKT/mTOR signalling and actin polymerisation in pulmonary veins of guinea pigs.Respir Res. 2018 Jun 19;19(1):120. doi: 10.1186/s12931-018-0829-5. Respir Res. 2018. PMID: 29921306 Free PMC article.
References
-
- AIT SAID F., WERTS C., ELALAMY I., COUETIL J.P., JACQUEMIN C., HATMI M. TNF-alpha, inefficient by itself, potentiates IL-1beta-induced PGHS-2 expression in human pulmonary microvascular endothelial cells: requirement of NF-kappaB and p38 MAPK pathways. Br. J. Pharmacol. 2002;136:1005–1014. - PMC - PubMed
-
- ALTIERE R.J., KIRITSY-ROY J.A., CATRAVAS J.D. Acetylcholine-induced contractions in isolated rabbit pulmonary arteries: role of thromboxane A2. J. Pharmacol. Exp. Ther. 1986;236:535–541. - PubMed
-
- ARNER M., HÖGESTÄTT E.D. Endothelium-dependent relaxation and effects of prostacyclin, endothelin and platelet-activating factor in human hand veins and arteries. Acta Physiol. Scand. 1991;142:165–172. - PubMed
-
- BÄCK M., WALCH L., NOREL X., GASCARD J.P., MAZMANIAN G., BRINK C. Modulation of vascular tone and reactivity by nitric oxide in porcine pulmonary arteries and veins. Acta Physiol. Scand. 2002;174:9–15. - PubMed
-
- BANSAL V., TOGA H., RAJ J.U. Tone dependent nitric oxide production in ovine vessels in vitro. Respir. Physiol. 1993;93:249–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

