Redox modulation of basal and beta-adrenergically stimulated cardiac L-type Ca(2+) channel activity by phenylarsine oxide
- PMID: 15172960
- PMCID: PMC1575054
- DOI: 10.1038/sj.bjp.0705845
Redox modulation of basal and beta-adrenergically stimulated cardiac L-type Ca(2+) channel activity by phenylarsine oxide
Abstract
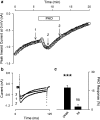
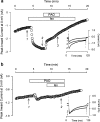
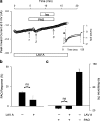
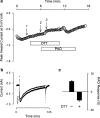
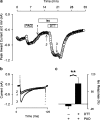
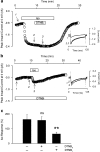
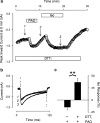
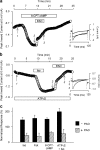
1. Phenylarsine oxide (PAO) is commonly used to inhibit tyrosine phosphatase activity. However, PAO can affect a variety of different processes because of its ability to promote sulfhydryl oxidation. In the present study, we investigated the effects that PAO has on basal and beta-adrenergically stimulated L-type Ca(2+) channel activity in isolated cardiac myocytes. 2. Extracellular application of PAO transiently stimulated the basal L-type Ca(2+) channel activity, whereas it irreversibly inhibited protein kinase A (PKA)-dependent regulation of channel activity by isoproterenol, forskolin and 8-CPT-cAMP (8-p-chlorophenylthioadenosine 3',5'-cyclic monophosphate). PAO also inhibited channel activity irreversibly stimulated in the presence of adenosine 5'-(3-thiotriphosphate) tetralithium salt. 3. Neither the stimulatory nor the inhibitory effects of PAO were affected by the tyrosine kinase inhibitor lavendustin A, suggesting that tyrosine phosphorylation is not involved. 4. Extracellular application of the sulfhydryl-reducing agent dithiothreitol (DTT) antagonized both the stimulatory and inhibitory effects of PAO. Yet, following intracellular dialysis with DTT, only the inhibitory effect of PAO was antagonized. 5. The inhibitory effect of PAO was mimicked by intracellular, but not extracellular application of the membrane impermeant thiol oxidant 5,5'-dithio-bis(2-nitrobenzoic acid). 6. These results suggest that the stimulatory effect of PAO results from oxidation of sulfhydryl residues at an extracellular site and the inhibitory effect is due to redox regulation of an intracellular site that affects the response of the channel to PKA-dependent phosphorylation. It is concluded that the redox state of the cell may play a critical role in modulating beta-adrenergic responsiveness of the L-type Ca(2+) channel in cardiac myocytes.
Figures
Similar articles
-
Tyrosine phosphatase inhibitors selectively antagonize beta-adrenergic receptor-dependent regulation of cardiac ion channels.Mol Pharmacol. 2000 Dec;58(6):1213-21. doi: 10.1124/mol.58.6.1213. Mol Pharmacol. 2000. PMID: 11093756
-
Role of tyrosine kinase activity in alpha-adrenergic inhibition of the beta-adrenergically regulated L-type Ca(2+) current in guinea-pig ventricular myocytes.J Physiol. 2001 Dec 15;537(Pt 3):779-92. doi: 10.1111/j.1469-7793.2001.00779.x. J Physiol. 2001. PMID: 11744754 Free PMC article.
-
Beta-3 adrenergic stimulation of L-type Ca(2+) channels in rat portal vein myocytes.Br J Pharmacol. 2000 Apr;129(7):1497-505. doi: 10.1038/sj.bjp.0703187. Br J Pharmacol. 2000. PMID: 10742307 Free PMC article.
-
Basal and β-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16598-603. doi: 10.1073/pnas.1419129111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368181 Free PMC article.
-
Protein tyrosine phosphorylation modulates microvessel permeability in frog mesentery.Microcirculation. 1996 Jun;3(2):245-7. doi: 10.3109/10739689609148297. Microcirculation. 1996. PMID: 8839449 Review. No abstract available.
Cited by
-
Pyruvate restores β-adrenergic sensitivity of L-type Ca(2+) channels in failing rat heart: role of protein phosphatase.Am J Physiol Heart Circ Physiol. 2013 May 15;304(10):H1352-60. doi: 10.1152/ajpheart.00873.2012. Epub 2013 Mar 15. Am J Physiol Heart Circ Physiol. 2013. PMID: 23504177 Free PMC article.
-
Oxidoreductase regulation of Kv currents in rat ventricle.J Mol Cell Cardiol. 2008 Jun;44(6):1062-1071. doi: 10.1016/j.yjmcc.2008.03.011. Epub 2008 Mar 28. J Mol Cell Cardiol. 2008. PMID: 18455732 Free PMC article.
-
Phosphatidylinositol-4,5-Biphosphate (PI(4,5)P2) Is Required for Rapid Endocytosis in Chromaffin Cells.Biomed Res Int. 2020 Sep 9;2020:9692503. doi: 10.1155/2020/9692503. eCollection 2020. Biomed Res Int. 2020. PMID: 32964048 Free PMC article.
-
Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2.Circ Res. 2008 May 9;102(9):e86-100. doi: 10.1161/CIRCRESAHA.108.173740. Epub 2008 Apr 24. Circ Res. 2008. PMID: 18436794 Free PMC article.
-
Redox Regulation of K+ Channel: Role of Thioredoxin.Antioxid Redox Signal. 2024 Nov;41(13-15):818-844. doi: 10.1089/ars.2023.0416. Epub 2024 Aug 28. Antioxid Redox Signal. 2024. PMID: 39099341 Free PMC article. Review.
References
-
- BELEVYCH A.E., WARRIER S., HARVEY R.D. Genistein inhibits cardiac L-type Ca2+ channel activity by a tyrosine kinase-independent mechanism. Mol. Pharmacol. 2002;62:554–565. - PubMed
-
- BELLES B., MALECOT C.O., HESCHELER J., TRAUTWEIN W. Run-down of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflügers Arch. 1988;411:353–360. - PubMed
-
- CHIAMVIMONVAT N., O'ROURKE B., KAMP T.J., KALLEN R.G., HOFMANN F., FLOCKERZI V., MARBAN E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ. Res. 1995;76:325–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

