Dynamics of putative raft-associated proteins at the cell surface
- PMID: 15173190
- PMCID: PMC2172371
- DOI: 10.1083/jcb.200312170
Dynamics of putative raft-associated proteins at the cell surface
Abstract
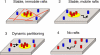
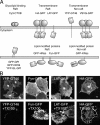
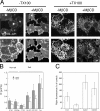
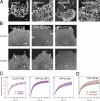
Lipid rafts are conceptualized as membrane microdomains enriched in cholesterol and glycosphingolipid that serve as platforms for protein segregation and signaling. The properties of these domains in vivo are unclear. Here, we use fluorescence recovery after photobleaching to test if raft association affects a protein's ability to laterally diffuse large distances across the cell surface. The diffusion coefficients (D) of several types of putative raft and nonraft proteins were systematically measured under steady-state conditions and in response to raft perturbations. Raft proteins diffused freely over large distances (> 4 microm), exhibiting Ds that varied 10-fold. This finding indicates that raft proteins do not undergo long-range diffusion as part of discrete, stable raft domains. Perturbations reported to affect lipid rafts in model membrane systems or by biochemical fractionation (cholesterol depletion, decreased temperature, and cholesterol loading) had similar effects on the diffusional mobility of raft and nonraft proteins. Thus, raft association is not the dominant factor in determining long-range protein mobility at the cell surface.
Copyright the Rockefeller University Press
Figures



References
-
- Anderson, R.G.W., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science. 296:1821–1825. - PubMed
-
- Aroeti, B., and Y.I. Henis. 1988. Effects of fusion temperature on the lateral mobility of Sendai virus glycoproteins in erythrocyte membranes and on cell fusion indicate that glycoprotein mobilization is required for cell fusion. Biochemistry. 27:5654–5661. - PubMed
-
- Brown, D.A., and J.K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68:533–544. - PubMed
-
- Brown, D.A., and E. London. 1998. a. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. - PubMed

