Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation
- PMID: 15173192
- PMCID: PMC2172388
- DOI: 10.1083/jcb.200309097
Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation
Abstract
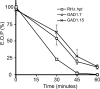
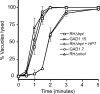
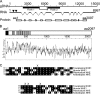
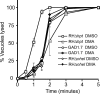
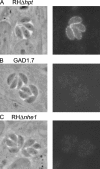
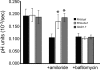
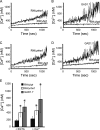
Calcium is a critical mediator of many intracellular processes in eukaryotic cells. In the obligate intracellular parasite Toxoplasma gondii, for example, a rise in [Ca2+] is associated with significant morphological changes and rapid egress from host cells. To understand the mechanisms behind such dramatic effects, we isolated a mutant that is altered in its responses to the Ca2+ ionophore A23187 and found the affected gene encodes a homologue of Na+/H+ exchangers (NHEs) located on the parasite's plasma membrane. We show that in the absence of TgNHE1, Toxoplasma is resistant to ionophore-induced egress and extracellular death and amiloride-induced proton efflux inhibition. In addition, the mutant has increased levels of intracellular Ca2+, which explains its decreased sensitivity to A23187. These results provide direct genetic evidence of a role for NHE1 in Ca2+ homeostasis and important insight into how this ubiquitous pathogen senses and responds to changes in its environment.
Copyright the Rockefeller University Press
Figures
References
-
- Berthe, P., J.L. Cousin, and J.P. Breittmayer. 1991. Intracellular Ca2+ regulation in CD3 stimulated Jurkat T cells involves H+ fluxes. Cell. Signal. 3:453–459. - PubMed
-
- Black, M.W., and J.C. Boothroyd. 1998. Development of a stable episomal shuttle vector for Toxoplasma gondii. J. Biol. Chem. 273:3972–3979. - PubMed
-
- Bosia, A., D. Ghigo, F. Turrini, E. Nissani, G.P. Pescarmona, and H. Ginsburg. 1993. Kinetic characterization of Na+/H+ antiport of Plasmodium falciparum membrane. J. Cell. Physiol. 154:527–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

