Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase
- PMID: 15173205
- PMCID: PMC2211776
- DOI: 10.1084/jem.20040382
Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase
Abstract
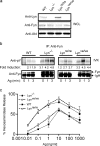
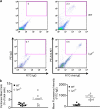
A role for Lyn kinase as a positive regulator of immunoglobulin (Ig)E-dependent allergy has long been accepted. Contrary to this belief, Lyn kinase was found to have an important role as a negative regulator of the allergic response. This became apparent from the hyperresponsive degranulation of lyn-/- bone marrow-derived mast cells, which is driven by hyperactivation of Fyn kinase that occurs, in part, through the loss of negative regulation by COOH-terminal Src kinase (Csk) and the adaptor, Csk-binding protein. This phenotype is recapitulated in vivo as young lyn-/- mice showed an enhanced anaphylactic response. In vivo studies also demonstrated that as lyn-/- mice aged, their serum IgE increased as well as occupancy of the high affinity IgE receptor (FcepsilonRI). This was mirrored by increased circulating histamine, increased mast cell numbers, increased cell surface expression of the high affinity IgE receptor (FcepsilonRI), and eosinophilia. The increased IgE production was not a consequence of increased Fyn kinase activity in lyn-/- mice because both lyn-/- and lyn-/- fyn-/- mice showed high IgE levels. Thus, lyn-/- mice and mast cells thereof show multiple allergy-associated traits, causing reconsideration of the possible efficacy in therapeutic targeting of Lyn in allergic disease.
Figures





References
-
- Field, K.A., D. Holowka, and B. Baird. 1997. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 272:4276–4280. - PubMed
-
- Rivera, J. 2002. Molecular adapters in FcɛRI signaling and the allergic response. Curr. Opin. Immunol. 14:688–693. - PubMed
-
- Nadler, M.J., S.A. Matthews, H. Turner, and J.-P. Kinet. 2000. Signal transduction by the high-affinity immunoglobulin E receptor Fc epsilon RI: coupling form to function. Adv. Immunol. 76:325–355. - PubMed
-
- Chan, V.W.F., F. Meng, P. Soriano, A.L. DeFranco, and C.A. Lowell. 1997. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 7:69–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

