Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells
- PMID: 15173207
- PMCID: PMC2211788
- DOI: 10.1084/jem.20032118
Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells
Abstract
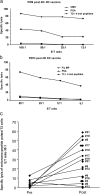
Cancer vaccines aim at inducing (a) tumor-specific effector T cells able to reduce/eliminate the tumor mass, and (b) long-lasting tumor-specific memory T cells able to control tumor relapse. We have shown earlier, in 18 human histocompatibility leukocyte antigen (HLA)-A*0201 patients with metastatic melanoma, that vaccination with peptide-loaded CD34-dendritic cells (DCs) leads to expansion of melanoma-specific interferon gamma-producing CD8+ T cells in the blood. Here, we show in 9 out of 12 analyzed patients the expansion of cytolytic CD8+ T cell precursors specific for melanoma differentiation antigens. These precursors yield, upon single restimulation with melanoma peptide-pulsed DCs, cytotoxic T lymphocytes (CTLs) able to kill melanoma cells. Melanoma-specific CTLs can be grown in vitro and can be detected in three assays: (a) melanoma tetramer binding, (b) killing of melanoma peptide-pulsed T2 cells, and (c) killing of HLA-A*0201 melanoma cells. The cytolytic activity of expanded CTLs correlates with the frequency of melanoma tetramer binding CD8+ T cells. Thus, CD34-DC vaccines can expand melanoma-specific CTL precursors that can kill melanoma antigen-expressing targets. These results justify the design of larger follow-up studies to assess the immunological and clinical response to peptide-pulsed CD34-DC vaccines.
Figures
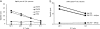


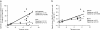
Similar articles
-
Novel approach to the characterization of melanoma associated-peptide-specific CTL lines from Japanese metastatic melanoma patients.Int J Oncol. 2008 Sep;33(3):433-41. Int J Oncol. 2008. PMID: 18695871
-
Boosting vaccinations with peptide-pulsed CD34+ progenitor-derived dendritic cells can expand long-lived melanoma peptide-specific CD8+ T cells in patients with metastatic melanoma.J Immunother. 2005 Mar-Apr;28(2):158-68. doi: 10.1097/01.cji.0000154249.74383.17. J Immunother. 2005. PMID: 15725960
-
Autologous dendritic cells derived from CD34+ progenitors and from monocytes are not functionally equivalent antigen-presenting cells in the induction of melan-A/Mart-1(27-35)-specific CTLs from peripheral blood lymphocytes of melanoma patients with low frequency of CTL precursors.Cancer Res. 1997 Dec 15;57(24):5534-41. Cancer Res. 1997. PMID: 9407964
-
Novel dendritic cell-based vaccination in late stage melanoma.Hum Vaccin Immunother. 2014;10(11):3132-8. doi: 10.4161/hv.29110. Hum Vaccin Immunother. 2014. PMID: 25483650 Free PMC article. Review.
-
Approaches to dendritic cell-based immunotherapy after peripheral blood stem cell transplantation.Ann N Y Acad Sci. 1999 Apr 30;872:363-71. doi: 10.1111/j.1749-6632.1999.tb08480.x. Ann N Y Acad Sci. 1999. PMID: 10372138 Review.
Cited by
-
NAFLD indirectly impairs antigen-specific CD8+ T cell immunity against liver cancer in mice.iScience. 2022 Feb 1;25(2):103847. doi: 10.1016/j.isci.2022.103847. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198900 Free PMC article.
-
Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial.Cell Mol Immunol. 2015 Jan;12(1):87-95. doi: 10.1038/cmi.2014.40. Epub 2014 Jun 30. Cell Mol Immunol. 2015. PMID: 24976269 Free PMC article. Clinical Trial.
-
Personalized dendritic cell-based tumor immunotherapy.Immunotherapy. 2010 Jan;2(1):57-68. doi: 10.2217/imt.09.78. Immunotherapy. 2010. PMID: 20161666 Free PMC article. Review.
-
Cell transfer therapy for cancer: past, present, and future.J Immunol Res. 2014;2014:525913. doi: 10.1155/2014/525913. Epub 2014 Jan 9. J Immunol Res. 2014. PMID: 24741604 Free PMC article. Review.
-
Trial watch: Dendritic cell-based interventions for cancer therapy.Oncoimmunology. 2012 Oct 1;1(7):1111-1134. doi: 10.4161/onci.21494. Oncoimmunology. 2012. PMID: 23170259 Free PMC article.
References
-
- Greenberg, P.D., J.P. Klarnet, D.E. Kern, and M.A. Cheever. 1988. Therapy of disseminated tumors by adoptive transfer of specifically immune T cells. Prog. Exp. Tumor Res. 32:104–127. - PubMed
-
- Herlyn, D., A. Linnenbach, H. Koprowski, and M. Herlyn. 1991. Epitope- and antigen-specific cancer vaccines. Int. Rev. Immunol. 7:245–257. - PubMed
-
- North, R.J. 1984. The murine antitumor immune response and its therapeutic manipulation. Adv. Immunol. 35:89–155. - PubMed
-
- Boon, T., J.C. Cerottini, B. Van den Eynde, P. van der Bruggen, and A. Van Pel. 1994. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12:337–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

