Surface mu heavy chain signals down-regulation of the V(D)J-recombinase machinery in the absence of surrogate light chain components
- PMID: 15173209
- PMCID: PMC2211789
- DOI: 10.1084/jem.20031523
Surface mu heavy chain signals down-regulation of the V(D)J-recombinase machinery in the absence of surrogate light chain components
Abstract
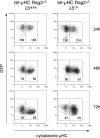
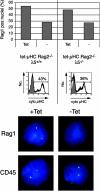
Early B cell development is characterized by stepwise, ordered rearrangement of the immunoglobulin (Ig) heavy (HC) and light (LC) chain genes. Only one of the two alleles of these genes is used to produce a receptor, a phenomenon referred to as allelic exclusion. It has been suggested that pre-B cell receptor (pre-BCR) signals are responsible for down-regulation of the VDJH-recombinase machinery (Rag1, Rag2, and terminal deoxynucleotidyl transferase [TdT]), thereby preventing further rearrangement on the second HC allele. Using a mouse model, we show that expression of an inducible muHC transgene in Rag2-/- pro-B cells induces down-regulation of the following: (a) TdT protein, (b) a transgenic green fluorescent protein reporter reflecting endogenous Rag2 expression, and (c) Rag1 primary transcripts. Similar effects were also observed in the absence of surrogate LC (SLC) components, but not in the absence of the signaling subunit Ig-alpha. Furthermore, in wild-type mice and in mice lacking either lambda5, VpreB1/2, or the entire SLC, the TdT protein is down-regulated in muHC+LC- pre-B cells. Surprisingly, muHC without LC is expressed on the surface of pro-/pre-B cells from lambda5-/-, VpreB1-/-VpreB2-/-, and SLC-/- mice. Thus, SLC or LC is not required for muHC cell surface expression and signaling in these cells. Therefore, these findings offer an explanation for the occurrence of HC allelic exclusion in mice lacking SLC components.
Figures



Similar articles
-
A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development.J Immunol. 1997 Aug 1;159(3):1265-75. J Immunol. 1997. PMID: 9233622
-
Only VpreB1, but not VpreB2, is expressed at levels which allow normal development of B cells.Int Immunol. 2006 Jan;18(1):163-72. doi: 10.1093/intimm/dxh359. Epub 2005 Dec 16. Int Immunol. 2006. PMID: 16361315
-
Down-regulation of terminal deoxynucleotidyl transferase by Ig heavy chain in B lineage cells.J Immunol. 1997 Feb 1;158(3):1133-8. J Immunol. 1997. PMID: 9013952
-
The pre-B-cell receptor.Curr Opin Immunol. 2007 Apr;19(2):137-42. doi: 10.1016/j.coi.2007.02.006. Epub 2007 Feb 15. Curr Opin Immunol. 2007. PMID: 17306522 Review.
-
The surrogate light chain in B-cell development.Immunol Today. 1993 Feb;14(2):60-8. doi: 10.1016/0167-5699(93)90060-X. Immunol Today. 1993. PMID: 8166770 Review.
Cited by
-
Agammaglobulinemia associated with BCR⁻ B cells and enhanced expression of CD19.Blood. 2011 Aug 18;118(7):1828-37. doi: 10.1182/blood-2011-01-330472. Epub 2011 Jun 21. Blood. 2011. PMID: 21693761 Free PMC article.
-
Human pre-B cell receptor signal transduction: evidence for distinct roles of PI3kinase and MAP-kinase signalling pathways.Immun Inflamm Dis. 2013 Oct;1(1):26-36. doi: 10.1002/iid3.4. Epub 2013 Oct 30. Immun Inflamm Dis. 2013. PMID: 25400915 Free PMC article.
-
In old BALB/c mice, bone marrow pre-B cell and surrogate light chain reduction is associated with increased B cell reactivity to phosphorylcholine, but reduced T15 idiotype dominance.Mech Ageing Dev. 2017 Mar;162:53-62. doi: 10.1016/j.mad.2016.11.004. Epub 2016 Nov 19. Mech Ageing Dev. 2017. PMID: 27876385 Free PMC article.
-
The pre-B-cell receptor induces silencing of VpreB and lambda5 transcription.EMBO J. 2005 Nov 16;24(22):3895-905. doi: 10.1038/sj.emboj.7600850. Epub 2005 Nov 10. EMBO J. 2005. PMID: 16281060 Free PMC article.
-
Mechanism for pre-B cell loss in VH-mutant rabbits.J Immunol. 2011 Nov 1;187(9):4714-20. doi: 10.4049/jimmunol.1101778. Epub 2011 Sep 28. J Immunol. 2011. PMID: 21957145 Free PMC article.
References
-
- Melchers, F., D. Haasner, U. Grawunder, C. Kalberer, H. Karasuyama, T. Winkler, and A.G. Rolink. 1994. Roles of IgH and L chains and of surrogate H and L chains in the development of cells of the B lymphocyte lineage. Annu. Rev. Immunol. 12:209–225. - PubMed
-
- Martensson, I.L., A. Rolink, F. Melchers, C. Mundt, S. Licence, and T. Shimizu. 2002. The pre-B cell receptor and its role in proliferation and Ig heavy chain allelic exclusion. Semin. Immunol. 14:335–342. - PubMed
-
- Grawunder, U., T.M. Leu, D.G. Schatz, A. Werner, A.G. Rolink, F. Melchers, and T.H. Winkler. 1995. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 3:601–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

