Plastidial alpha-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress
- PMID: 15173560
- PMCID: PMC514120
- DOI: 10.1104/pp.103.032631
Plastidial alpha-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress
Abstract
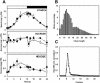
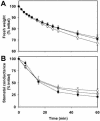
To study the role of the plastidial alpha-glucan phosphorylase in starch metabolism in the leaves of Arabidopsis, two independent mutant lines containing T-DNA insertions within the phosphorylase gene were identified. Both insertions eliminate the activity of the plastidial alpha-glucan phosphorylase. Measurement of other enzymes of starch metabolism reveals only minor changes compared with the wild type. The loss of plastidial alpha-glucan phosphorylase does not cause a significant change in the total accumulation of starch during the day or its remobilization at night. Starch structure and composition are unaltered. However, mutant plants display lesions on their leaves that are not seen on wild-type plants, and mesophyll cells bordering the lesions accumulate high levels of starch. Lesion formation is abolished by growing plants under 100% humidity in still air, but subsequent transfer to circulating air with lower humidity causes extensive wilting in the mutant leaves. Wilted sectors die, causing large lesions that are bordered by starch-accumulating cells. Similar lesions are caused by the application of acute salt stress to mature plants. We conclude that plastidial phosphorylase is not required for the degradation of starch, but that it plays a role in the capacity of the leaf lamina to endure a transient water deficit.
Figures
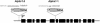

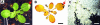


References
-
- Albrecht T, Koch A, Lode A, Greve B, Schneider-Mergener J, Steup M (2001) Plastidic (Pho1-type) phosphorylase isoforms in potato (Solanum tuberosum L.) plants: expression analysis and immunochemical characterisation. Planta 213: 602–613 - PubMed
-
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316: 1194–1199
-
- Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40: 95–117
-
- Bouchez D, Camilleri C, Caboche M (1993) A binary vector based on Basta resistance for in planta transformation of Arabidopsis thaliana. C R Acad Sci Paris Life Sci 316: 1188–1193
-
- Buchner P, Borisjuk L, Wobus U (1996) Glucan phosphorylases in Vicia faba L.: cloning, structural analysis and expression patterns of cytosolic and plastidic forms in relation to starch. Planta 199: 64–73 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

