beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha
- PMID: 15173580
- PMCID: PMC423241
- DOI: 10.1073/pnas.0402851101
beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha
Abstract
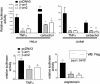
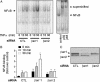
In addition to their roles in desensitization and signaling of seven-membrane-spanning receptors, beta-arrestins have been more recently implicated in regulating non-seven-membrane-spanning receptor pathways. By using a yeast two-hybrid screen, we identified the inhibitor of NF-kappaB, IkappaBalpha, as a binding partner of beta-arrestin 1. Both beta-arrestin 1 and 2 interact with IkappaBalpha in transfected cells as assessed by immunoprecipitation experiments. Additionally, upstream kinases known to regulate the function of IkappaBalpha, such as IkappaB kinase alpha and beta and NF-kappaB-inducing kinase, were also shown to interact with beta-arrestin. Overexpression of either beta-arrestin 1 or beta-arrestin 2 led to marked inhibition of NF-kappaB activity, as measured by reporter gene activity. Inhibition of NF-kappaB activity was independent of the type of stimulus used for NF-kappaB activation. Conversely, suppression of beta-arrestin 1, but not beta-arrestin 2, expression by using RNA interference led to a 3-fold increase in tumor necrosis factor-stimulated NF-kappaB activity as measured by NF-kappaB mobility-shift analysis. These data uncover a role of beta-arrestins in the regulation of NF-kappaB-mediated gene regulation.
Figures


References
-
- Lohse, M. J., Benovic, J. L., Codina, J., Caron, M. G. & Lefkowitz, R. J. (1990) Science 248, 1547–1550. - PubMed
-
- Attramadal, H., Arriza, J. L., Aoki, C., Dawson, T. M., Codina, J., Kwatra, M. M., Snyder, S. H., Caron, M. G. & Lefkowitz, R. J. (1992) J. Biol. Chem. 267, 17882–17890. - PubMed
-
- Krupnick, J. G. & Benovic, J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289–319. - PubMed
-
- Zhang, J., Ferguson, S. S., Barak, L. S., Aber, M. J., Giros, B., Lefkowitz, R. J. & Caron, M. G. (1997) Receptors Channels 5, 193–199. - PubMed
-
- Lefkowitz, R. J. (1998) J. Biol. Chem. 273, 18677–18680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

