Total synthesis of (-)-spinosyn A
- PMID: 15173590
- PMCID: PMC514415
- DOI: 10.1073/pnas.0401247101
Total synthesis of (-)-spinosyn A
Abstract
A convergent, highly stereoselective total synthesis of (-)-spinosyn A (1) is described. Key features of the synthesis include the transannular Diels-Alder reaction of macrocyclic pentaene 11 and the transannular Morita-Baylis-Hillman cyclization of 12 that generates tetracycle 26. The total synthesis of (-)-spinosyn A was completed by a sequence involving the highly beta-selective glycosidation reaction of 13 and glycosyl imidate 30.
Figures
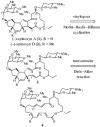
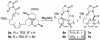

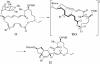
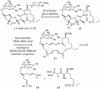
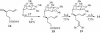
 CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.
CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.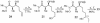
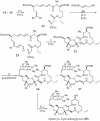
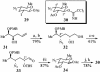
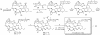
References
-
- Kirst, H. A., Michel, K. H., Martin, J. W., Creemer, L. C., Chio, E. H., Yao, R. C., Nakatsukasa, W. M., Boeck, L., Occolowitz, J. L., Paschal, J. W., et al. (1991) Tetrahedron Lett. 32, 4839-4842.
-
- Kirst, H. A., Michel, K. H., Chio, E. H., Yao, R. C., Nakatsukasa, W. M., Boeck, L., Occolowitz, J. L., Paschal, J. W., Deeter, J. B. & Thompson, G. D. (1991) in Microbial Metabolites, ed. Nash, C. (William C. Brown, Dubuque, IA), Vol. 32, pp. 109.
-
- Kirst, H. A., Michel, K. H., Mynderase, J. S., Chio, E. H., Yao, R. C., Nakasukasa, W. M., Boeck, L. D., Occlowitz, J. L., Paschal, J. W., Deeter, J. B., et al. (1992) in Synthesis and Chemistry of Agrochemicals III, eds. Baker, D. R., Fenyes, J. G. & Steffens, J. J. (Am. Chem. Soc., Washington, DC), Vol. ACS Symposium Series 504, pp. 214-225.
-
- Martynow, J. G. & Kirst, H. A. (1994) J. Org. Chem. 59, 1548-1560.
-
- Creemer, L. C., Kirst, H. A. & Paschal, J. W. (1998) J. Antibiot. (Tokyo) 51, 795-800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

