Bilayers merge even when exocytosis is transient
- PMID: 15173592
- PMCID: PMC423272
- DOI: 10.1073/pnas.0401316101
Bilayers merge even when exocytosis is transient
Abstract
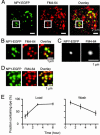
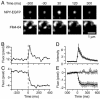
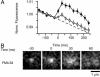
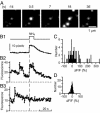
During exocytosis, the lumen of secretory vesicles connects with the extracellular space. In some vesicles, this connection closes again, causing the vesicle to be recaptured mostly intact. The degree to which the bilayers of such vesicles mix with the plasma membrane is unknown. Work supporting the kiss-and-run model of transient exocytosis implies that synaptic vesicles allow neither lipid nor protein to escape into the plasma membrane, suggesting that the two bilayers never merge. Here, we test whether neuroendocrine granules behave similarly. Using two-color evanescent field microscopy, we imaged the lipid probe FM4-64 and fluorescent proteins in single dense core granules. During exocytosis, granules lost FM4-64 into the plasma membrane in small fractions of a second. Although FM4-64 was lost, granules retained the membrane protein, GFP-phogrin. By using GFP-phogrin as a probe for resealing, it was found that even granules that reseal lose FM4-64. We conclude that the lipid bilayers of the granule and the plasma membrane become continuous even when exocytosis is transient.
Figures

References
-
- Fernandez, J. M., Neher, E. & Gomperts, B. D. (1984) Nature 312, 453–455. - PubMed
-
- Spruce, A. E., Breckenridge, L. J., Lee, A. K. & Almers, W. (1990) Neuron 4, 643–654. - PubMed
-
- Albillos, A., Dernick, G., Horstmann, H., Almers, W., Alvarez de Toledo, G. & Lindau, M. (1997) Nature 389, 509–512. - PubMed
-
- Ales, E., Tabares, L., Poyato, J. M., Valero, V., Lindau, M. & Alvarez de Toledo, G. (1999) Nat. Cell Biol. 1, 40–44. - PubMed
-
- Angleson, J. K., Cochilla, A. J., Kilic, G., Nussinovitch, I. & Betz, W. J. (1999) Nat. Neurosci. 2, 440–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

