Development of antigen-specific ELISA for circulating autoantibodies to extracellular matrix protein 1 in lichen sclerosus
- PMID: 15173881
- PMCID: PMC419485
- DOI: 10.1172/JCI20373
Development of antigen-specific ELISA for circulating autoantibodies to extracellular matrix protein 1 in lichen sclerosus
Abstract
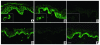
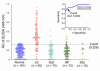
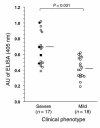
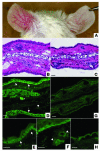
Lichen sclerosus is a common, acquired chronic inflammatory skin disease of unknown etiology, although circulating autoantibodies to the glycoprotein extracellular matrix protein 1 (ECM1) have been detected in most patients' sera. We have examined the nature of ECM1 epitopes in lichen sclerosus sera, developed an ELISA system for serologic diagnosis, and assessed clinicopathological correlation between ELISA titer and disease. Epitope-mapping studies revealed that lichen sclerosus sera most frequently recognized the distal second tandem repeat domain and carboxyl-terminus of ECM1. We analyzed serum autoantibody reactivity against this immunodominant epitope in 413 individuals (95 subjects with lichen sclerosus, 161 normal control subjects, and 157 subjects with other autoimmune basement membrane or sclerosing diseases). The ELISA assay was highly sensitive; 76 of 95 lichen sclerosus patients (80.0%) exhibited IgG reactivity. It was also highly specific (93.7%) in discriminating between lichen sclerosus and other disease/control sera. Higher anti-ECM1 titers also correlated with more longstanding and refractory disease and cases complicated by squamous cell carcinoma. Furthermore, passive transfer of affinity-purified patient IgG reproduced some histologic and immunopathologic features of lichen sclerosus skin. This new ELISA is valuable for the accurate detection and quantification of anti-ECM1 autoantibodies. Moreover, the values may have clinical significance in patients with lichen sclerosus.
Figures



Similar articles
-
Characterization of IgG autoantibodies to extracellular matrix protein 1 in lichen sclerosus.Clin Exp Dermatol. 2004 Sep;29(5):499-504. doi: 10.1111/j.1365-2230.2004.01573.x. Clin Exp Dermatol. 2004. PMID: 15347336
-
Autoantibodies to extracellular matrix protein 1 in lichen sclerosus.Lancet. 2003 Jul 12;362(9378):118-23. doi: 10.1016/S0140-6736(03)13863-9. Lancet. 2003. PMID: 12867112
-
Three-dimensional imaging reveals major changes in skin microvasculature in lipoid proteinosis and lichen sclerosus.J Dermatol Sci. 2005 Jun;38(3):215-24. doi: 10.1016/j.jdermsci.2005.01.012. Epub 2005 Mar 3. J Dermatol Sci. 2005. PMID: 15927815
-
The role of extracellular matrix protein 1 in human skin.Clin Exp Dermatol. 2004 Jan;29(1):52-6. doi: 10.1111/j.1365-2230.2004.01440.x. Clin Exp Dermatol. 2004. PMID: 14723723 Review.
-
[Lichen sclerosus. New aspects of pathogenesis and treatment].Hautarzt. 2005 Jun;56(6):550-5. doi: 10.1007/s00105-005-0955-0. Hautarzt. 2005. PMID: 15889230 Review. German.
Cited by
-
Pediatric Lichen Sclerosus: A Review of the Literature and Management Recommendations.J Clin Aesthet Dermatol. 2016 Sep;9(9):49-54. Epub 2016 Sep 1. J Clin Aesthet Dermatol. 2016. PMID: 27878062 Free PMC article. Review.
-
Alteration of gene expression related to vulvar smooth muscle, extracellular matrix and innervation in vulvar lichen sclerosus: A pilot study.Health Sci Rep. 2020 Nov 27;3(4):e208. doi: 10.1002/hsr2.208. eCollection 2020 Dec. Health Sci Rep. 2020. PMID: 33313423 Free PMC article. No abstract available.
-
Unusual remodeling of the hyalinization band in vulval lichen sclerosus by type V collagen and ECM 1 protein.Clinics (Sao Paulo). 2015 May;70(5):356-62. doi: 10.6061/clinics/2015(05)09. Epub 2015 May 1. Clinics (Sao Paulo). 2015. PMID: 26039953 Free PMC article.
-
The co-occurrence of lichen sclerosus et atrophicus and celiac disease.Indian Dermatol Online J. 2014 Dec;5(Suppl 2):S106-8. doi: 10.4103/2229-5178.146172. Indian Dermatol Online J. 2014. PMID: 25593796 Free PMC article.
-
Evaluation and characterization of anti-RalA autoantibody as a potential serum biomarker in human prostate cancer.Oncotarget. 2016 Jul 12;7(28):43546-43556. doi: 10.18632/oncotarget.9869. Oncotarget. 2016. PMID: 27286458 Free PMC article.
References
-
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J. Am. Acad. Dermatol. 1995;32:393–416. - PubMed
-
- Tasker GL, Wojnarowska F. Lichen sclerosus. Clin. Exp. Dermatol. 2003;28:128–133. - PubMed
-
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777–1783. - PubMed
-
- Meyrick-Thomas RH, Ridley CM, McGibbon DH, Black MM. Lichen sclerosus et atrophicus and autoimmunity—a study of 350 women. Br. J. Dermatol. 1998;118:41–46. - PubMed
-
- Wallace HJ. Lichen sclerosus et atrophicus. Trans. St. Johns. Hosp. Dermatol. Soc. 1971;57:9–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

