Concentration-dependent regulation of thyrotropin receptor function by thyroid-stimulating antibody
- PMID: 15173885
- PMCID: PMC419493
- DOI: 10.1172/JCI21334
Concentration-dependent regulation of thyrotropin receptor function by thyroid-stimulating antibody
Abstract
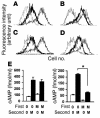
Thyrotropin receptor (TSHR) Ab's of the stimulating variety are the cause of hyperthyroid Graves disease. MS-1 is a hamster mAb with TSHR-stimulating activity. To examine the in vivo biological activity of MS-1, mice were treated with purified MS-1 intraperitoneally and the thyroid response evaluated. MS-1 induced a dose-dependent increase in serum thyroxine (T4), with a maximum effect after 10 proportional, variant g of MS-1 was administered. MS-1-secreting hybridoma cells were then transferred into the peritoneum of nude mice to study chronic thyroid stimulation. Serum MS-1 levels detected after 2 weeks were approximately 10-50 proportional, variant g/ml, and the serum TSH was suppressed in all animals. Serum triiodothyronine levels were elevated, but only in animals with low serum MS-1 concentrations. In addition, there was a negative correlation between serum T4 and the serum MS-1 concentrations. These in vivo studies suggested a partial TSHR inactivation induced by excessive stimulation by MS-1. We confirmed this inactivation by demonstrating MS-1 modulation of TSHR function in vitro as evidenced by downregulation and desensitization of the TSHR at concentrations of MS-1 achieved in the in vivo studies. Thus, inactivation of the TSHR by stimulating TSHR autoantibodies (TSHR-Ab's) in Graves disease patients may provide a functional explanation for the poor correlation between thyroid function and serum TSHR-Ab concentrations.
Figures
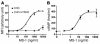



References
-
- Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr. Rev. 1988;9:106–121. - PubMed
-
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr. Rev. 1998;19:673–716. - PubMed
-
- Adams DD, Purves HD. Abnormal responses in the assay of thyrotropins. Proc. Univ. Otago Med. Sch. 1956;34:11–12.
-
- Kriss J, Pleshakov V, Chien JR. Isolation and identification of the long acting thyroid stimulator and its relation to hyperthyroidism and circumscribed pretibial myxedema. J. Clin. Endocrinol. Metab. 1964;24:1005–1028. - PubMed

