Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response
- PMID: 15173891
- PMCID: PMC419481
- DOI: 10.1172/JCI18704
Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response
Abstract
While the initiation of the adaptive and innate immune response is well understood, less is known about cellular mechanisms propagating inflammation. The receptor for advanced glycation end products (RAGE), a transmembrane receptor of the immunoglobulin superfamily, leads to perpetuated cell activation. Using novel animal models with defective or tissue-specific RAGE expression, we show that in these animal models RAGE does not play a role in the adaptive immune response. However, deletion of RAGE provides protection from the lethal effects of septic shock caused by cecal ligation and puncture. Such protection is reversed by reconstitution of RAGE in endothelial and hematopoietic cells. These results indicate that the innate immune response is controlled by pattern-recognition receptors not only at the initiating steps but also at the phase of perpetuation.
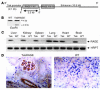
Figures







References
-
- Medzhitov R, Preston Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Modlin RL, Brightbill HD, Godowski PJ. The toll of innate immunity on microbial pathogens. N. Engl. J. Med. 1999;340:1834–1835. - PubMed
-
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins. Evolutionary conserved mediators of immune response. Annu. Rev. Immunol. 1998;16:225–260. - PubMed
-
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

