The IL-12Rbeta2 gene functions as a tumor suppressor in human B cell malignancies
- PMID: 15173892
- PMCID: PMC419484
- DOI: 10.1172/JCI20303
The IL-12Rbeta2 gene functions as a tumor suppressor in human B cell malignancies
Retraction in
-
Retraction. The IL-12Rβ2 gene functions as a tumor suppressor in human B cell malignancies.J Clin Invest. 2014 Jun;124(6):2807. doi: 10.1172/JCI76855. Epub 2014 Jun 2. J Clin Invest. 2014. PMID: 24892713 Free PMC article. No abstract available.
Abstract
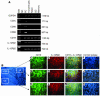
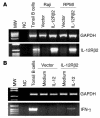
The IL-12Rbeta2 gene is expressed in human mature B cell subsets but not in transformed B cell lines. Silencing of this gene may be advantageous to neoplastic B cells. Our objective was to investigate the mechanism(s) and the functional consequence(s) of IL-12Rbeta2 gene silencing in primary B cell tumors and transformed B cell lines. Purified tumor cells from 41 patients with different chronic B cell lymphoproliferative disorders, representing the counterparts of the major mature human B cell subsets, tested negative for IL-12Rbeta2 gene expression. Hypermethylation of a CpG island in the noncoding exon 1 was associated with silencing of this gene in malignant B cells. Treatment with the DNA methyltransferase inhibitor 5-Aza-2'-deoxycytidine restored IL-12Rbeta2 mRNA expression in primary neoplastic B cells that underwent apoptosis following exposure to human recombinant IL-12 (hrIL-12). hrIL-12 inhibited proliferation and increased the apoptotic rate of IL-12Rbeta2-transfected B cell lines in vitro. Finally, hrIL-12 strongly reduced the tumorigenicity of IL-12Rbeta2-transfected Burkitt lymphoma RAJI cells in SCID-NOD mice through antiproliferative and proapoptotic effects, coupled with neoangiogenesis inhibition related to human IFN-gamma-independent induction of hMig/CXCL9. The IL-12Rbeta2 gene acts as tumor suppressor in chronic B cell malignancies, and IL-12 exerts direct antitumor effects on IL-12Rbeta2-expressing neoplastic B cells.
Figures
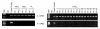


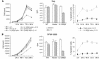

Similar articles
-
Methylation of the IL-12Rbeta2 gene as novel tumor escape mechanism for pediatric B-acute lymphoblastic leukemia cells.Cancer Res. 2006 Apr 15;66(8):3978-80. doi: 10.1158/0008-5472.CAN-05-4418. Cancer Res. 2006. PMID: 16618714
-
Heterogeneous expression of interleukin-18 and its receptor in B-cell lymphoproliferative disorders deriving from naive, germinal center, and memory B lymphocytes.Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):144-54. doi: 10.1158/1078-0432.ccr-1026-3. Clin Cancer Res. 2004. PMID: 14734463
-
Corrective effects of interleukin-12 on age-related deficiencies in IFN-gamma production and IL-12Rbeta2 expression in virus-specific CD8+ T cells.J Interferon Cytokine Res. 2000 Feb;20(2):235-45. doi: 10.1089/107999000312658. J Interferon Cytokine Res. 2000. PMID: 10714560
-
The interleukin-12 and interleukin-12 receptor system in normal and transformed human B lymphocytes.Haematologica. 2002 Apr;87(4):434-42. Haematologica. 2002. PMID: 11940489 Review.
-
Interleukin-12 receptor beta2: from cytokine receptor to gatekeeper gene in human B-cell malignancies.J Clin Oncol. 2009 Oct 1;27(28):4809-16. doi: 10.1200/JCO.2008.21.3579. Epub 2009 Aug 31. J Clin Oncol. 2009. PMID: 19720917 Review.
Cited by
-
Tumor suppressor gene methylation in follicular lymphoma: a comprehensive review.Mol Cancer. 2006 Oct 6;5:44. doi: 10.1186/1476-4598-5-44. Mol Cancer. 2006. PMID: 17026765 Free PMC article. Review.
-
Type I cytokine profiles of human naïve and memory B lymphocytes: a potential for memory cells to impact polarization.Immunology. 2006 May;118(1):66-77. doi: 10.1111/j.1365-2567.2006.02342.x. Immunology. 2006. PMID: 16630024 Free PMC article.
-
Identification of a genetic locus for autosomal dominant disseminated superficial actinic porokeratosis on chromosome 1p31.3-p31.1.Hum Genet. 2008 Jun;123(5):507-13. doi: 10.1007/s00439-008-0504-x. Epub 2008 Apr 29. Hum Genet. 2008. PMID: 18443824
-
Identification and expression analysis of alternatively spliced isoforms of human interleukin-23 receptor gene in normal lymphoid cells and selected tumor cells.Immunogenetics. 2006 Jan;57(12):934-43. doi: 10.1007/s00251-005-0067-0. Epub 2005 Dec 22. Immunogenetics. 2006. PMID: 16372191
-
IL-12 can target human lung adenocarcinoma cells and normal bronchial epithelial cells surrounding tumor lesions.PLoS One. 2009 Jul 1;4(7):e6119. doi: 10.1371/journal.pone.0006119. PLoS One. 2009. PMID: 19582164 Free PMC article.
References
-
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. - PubMed
-
- Perussia B, et al. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J. Immunol. 1992;149:3495–3502. - PubMed
-
- Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J. Immunol. 2000;165:2665–2670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

