HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis
- PMID: 15175247
- PMCID: PMC5142244
- DOI: 10.1242/dev.01187
HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis
Abstract
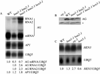
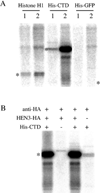
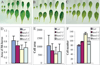
In plants, organs are generated post-embryonically from highly organized structures known as meristems. Cell division in the meristem is closely integrated with cell fate specification and organ formation. The presence of multiple cyclin-dependent kinases (CDKs) and their partner cyclins in plants and other multicellular organisms probably reflects the complexity of cell cycle regulation within developmental contexts. The Arabidopsis genome encodes at least eight CDKs and 30 cyclins. However, no mutants in any CDKs have been reported, and the function of the great majority of these genes in plant development is unknown. We show that HUA ENHANCER3 (HEN3), which encodes CDKE, a homolog of mammalian CDK8, is required for the specification of stamen and carpel identities and for the proper termination of stem cells in the floral meristem. Therefore, CDK8 plays a role in cell differentiation in a multicellular organism.
Figures



Similar articles
-
ULTRAPETALA1 encodes a SAND domain putative transcriptional regulator that controls shoot and floral meristem activity in Arabidopsis.Development. 2005 Mar;132(5):897-911. doi: 10.1242/dev.01642. Epub 2005 Jan 26. Development. 2005. PMID: 15673576
-
The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1.Development. 2004 Nov;131(22):5649-57. doi: 10.1242/dev.01441. Development. 2004. PMID: 15509765
-
Molecular characterization of Arabidopsis PHO80-like proteins, a novel class of CDKA;1-interacting cyclins.Cell Mol Life Sci. 2004 Jun;61(12):1485-97. doi: 10.1007/s00018-004-4057-4. Cell Mol Life Sci. 2004. PMID: 15197472 Free PMC article.
-
Coming into bloom: the specification of floral meristems.Development. 2009 Oct;136(20):3379-91. doi: 10.1242/dev.033076. Development. 2009. PMID: 19783733 Review.
-
Control of floral stem cell activity in Arabidopsis.Plant Signal Behav. 2019;14(11):1659706. doi: 10.1080/15592324.2019.1659706. Epub 2019 Aug 29. Plant Signal Behav. 2019. PMID: 31462133 Free PMC article. Review.
Cited by
-
CYCLIN-DEPENDENT KINASE G1 is associated with the spliceosome to regulate CALLOSE SYNTHASE5 splicing and pollen wall formation in Arabidopsis.Plant Cell. 2013 Feb;25(2):637-48. doi: 10.1105/tpc.112.107896. Epub 2013 Feb 12. Plant Cell. 2013. PMID: 23404887 Free PMC article.
-
Functional evolution of cyclin-dependent kinases.Mol Biotechnol. 2009 May;42(1):14-29. doi: 10.1007/s12033-008-9126-8. Epub 2009 Jan 15. Mol Biotechnol. 2009. PMID: 19145493 Review.
-
Mapping the Influence of Light Intensity on the Transgenerational Genetic Architecture of Arabidopsis thaliana.Curr Issues Mol Biol. 2024 Jul 29;46(8):8148-8169. doi: 10.3390/cimb46080482. Curr Issues Mol Biol. 2024. PMID: 39194699 Free PMC article.
-
Diversity, classification and function of the plant protein kinase superfamily.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 19;367(1602):2619-39. doi: 10.1098/rstb.2012.0003. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22889912 Free PMC article.
-
Defects in Cell Wall Differentiation of the Arabidopsis Mutant rol1-2 Is Dependent on Cyclin-Dependent Kinase CDK8.Cells. 2021 Mar 19;10(3):685. doi: 10.3390/cells10030685. Cells. 2021. PMID: 33808926 Free PMC article.
References
-
- Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–106. - PubMed
-
- Boniotti MB, Gutierrez C. A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. Plant J. 2001;28:341–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

