Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells
- PMID: 15175261
- PMCID: PMC420355
- DOI: 10.1101/gad.1209204
Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells
Abstract
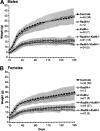
Homologous recombination (HR) and nonhomologous end-joining (NHEJ) are mechanistically distinct DNA repair pathways that contribute substantially to double-strand break (DSB) repair in mammalian cells. We have combined mutations in factors from both repair pathways, the HR protein Rad54 and the DNA-end-binding factor Ku80, which has a role in NHEJ. Rad54(-/-)Ku80(-/-) mice were severely compromised in their survival, such that fewer double mutants were born than expected, and only a small proportion of those born reached adulthood. However, double-mutant mice died at lower frequency from tumors than Ku80 single mutant mice, likely as a result of rapid demise at a young age from other causes. When challenged with an exogenous DNA damaging agent, ionizing radiation, double-mutant mice were exquisitely sensitive to low doses. Tissues and cells from double-mutant mice also showed indications of spontaneous DNA damage. Testes from some Rad54(-/-)Ku80(-/-) mice displayed enhanced apoptosis and reduced sperm production, and embryonic fibroblasts from Rad54(-/-)Ku80(-/-) animals accumulated foci of gamma-H2AX, a marker for DSBs. The substantially increased DNA damage response in the double mutants implies a cooperation of the two DSB repair pathways for survival and genomic integrity in the animal.
Figures


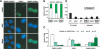


References
-
- Cromie, G.A., Connelly, J.C., and Leach, D.R. 2001. Recombination at double-strand breaks and DNA ends: Conserved mechanisms from phage to humans. Mol. Cell 8: 1163-1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
